Rationale and design of a study to test the effectiveness of a combined community health worker and text messaging-based intervention for smoking cessation in India (Project MUKTI)
Introduction
Tobacco use contributes to more than a million deaths in India annually, ranking as the second leading cause of disability adjusted life years (DALYs) in the country (1,2). Moreover, unlike high income countries, the prevalence of tobacco use continues to rise, especially in young people (3). Other low- and middle-income countries suffer a similar situation (4). Providing adequate smoking cessation services is a part of the MPOWER package, which is a set of six measures advocated by the World Health Organization (WHO) to reduce tobacco use globally (5). While India has made progress in regulating tobacco (by signing the WHO framework convention on tobacco control (FCTC), amongst others), provision of smoking cessation services to tobacco users has been wholly inadequate (6).
There is, therefore, an urgent need to develop models of tobacco cessation treatment that are effective, inexpensive, scalable and culturally acceptable. Community health workers (CHWs) have a successful track record in improving maternal health and various communicable disease imperatives (7). CHWs are lay community members who are trained in specific areas, and are primarily aimed at bridging the gap between traditional healthcare providers and patients. More recently, they have been used in noncommunicable diseases such as hypertension and diabetes with encouraging results, raising the question whether they could be effective in promoting quitting from tobacco (8). A brief community outreach intervention delivered by CHWs has previously been shown to increase tobacco cessation in India (9). However, the effect size was small. We have previously tested a CHW based approach to providing smoking cessation support for tobacco users in their homes (10). The study was based on the transtheoretical model of change, with contemplative participants receiving more intensive intervention from CHWs, as compared to precontemplative participants. At the end of 2 years of intervention, there was no significant difference between the intervention and control groups (11). Given that prior literature shows a direct relationship between the dose of a tobacco cessation intervention and results, we posited that inadequate hours of patient contact with CHWs may have partly driven the null findings. Since the ability of CHWs to spend time on tobacco related activities is finite, we hypothesized that supplementing a CHW led approach to smoking cessation with text messaging through a mobile device may lead to increased rates of quitting tobacco. Previous studies have shown that text messaging-based solutions can improve quit rates of tobacco use in various populations (12). However, effectiveness of text messaging for smoking cessation has mostly been evaluated in high income countries and include only those who are contemplating quitting (13). While the transtheoretical model provides a construct to frame the intervention, traditional interventions have not achieved much success with precontemplative participants. Therefore, employing motivational interviewing techniques for precontemplative participants may help a greater proportion progress to the contemplative stage (14). Multiple studies have shown that motivational interviewing can increase quit rates of tobacco use, though no study has specifically employed CHWs to deliver motivational interviewing (15). Hence, tailoring the appropriate intervention to an individual’s stage of change can potentially result in higher quit rates.
We therefore hypothesized that a home based CHW led approach to smoking cessation, incorporating motivational interviewing and accompanied by text messaging, would lead to increased quit rates amongst users of smoked tobacco in India.
Methods
The design, implementation and reporting of the study follows recommendations from the ‘Ottawa Statement on Ethical Design and Conduct of Cluster Randomized Trials’ (16). The study protocol received approval from the institutional review board of University Hospitals Cleveland Medical Center, USA and Society for the Promotion of Ethical Clinical Trials (SPECT), an independent review committee in New Delhi, India. All trial participants provided written informed consent and the trial was registered in the clinicaltrials.gov database (registration number- NCT03495622).
Study design
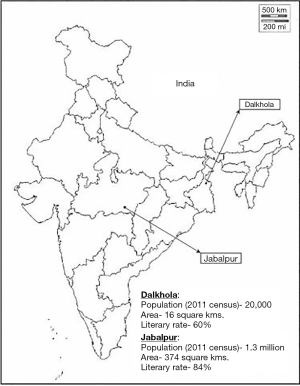
Project multi-unit kit for tobacco intervention (MUKTI) is a two armed, parallel, cluster randomized controlled trial conducted in two sites in India—Dalkhola and Jabalpur (Figure 1). Dalkhola is a town in eastern India, with a population of around 20,000 people and a literacy rate of 60%. Jabalpur, on the other hand, is a city in central India, with a population of 1.2 million and a literacy rate of 84%. The average national literacy rate is 74% (17).
Study population
Adults aged 18 to 70 years, who live in either of the two study sites and smoke daily, will be recruited into the study by the CHW. Home based screening will be conducted by the CHW assigned to each geographical cluster, using the voter list of the area as a guide. Among the screened individuals, those who smoke cigarettes or bidis daily (self-reported) and have access to a mobile phone will be recruited into the study. Bidis are inexpensive, hand rolled cigarettes commonly used in India. Screening will continue until she reaches the target study population of 35 participants in each cluster.
Exclusion criteria include those who are bed-bound because of an acute illness or a chronic condition. Participants who have significant disabilities that would prevent them from participating in the study in a meaningful way such as being blind, deaf or intellectually disabled will be excluded from the study. Illiterate participants will not be excluded, as long as they have access to a cellphone and a family member who can read out text messages to them. The CHW will have discretionary power to exclude any individual who may not be able to participate in the study and will also exclude those who may not be available for follow up in the following year.
Randomization
Randomization will be done at the level of the geographical cluster at the 2 sites. Dalkhola and Jabalpur are geographically divided into 14 and 30 wards, respectively. Each ward consists of smaller regions. Using the voter list as a reference, these regions are combined to form clusters of approximately equal population sizes. We will select a total of 16 clusters for the study, 12 from Dalkhola and 4 from Jabalpur. From these 16 clusters, 8 clusters will be randomly assigned to the intervention group, and 8 clusters assigned to the control group. A computer-generated sequence will be used for randomization. Post randomization, if a randomly selected control cluster is adjacent to an intervention cluster, we will exclude the control cluster and randomly choose a new control cluster from the remaining clusters. This is done to avoid contamination of the control group. Once the cluster randomization is finalized, CHWs will start the screening process.
The community health worker
The role of CHWs in controlling non-communicable diseases is a growing area of interest but their utility in smoking cessation interventions is yet to be fully defined. Furthermore, their ability to utilize motivational interviewing to elicit change has not been previously studied. We have previously described our selection and training processes for CHWs (18). Briefly, our CHWs are recruited through a written test and interview. Once selected, they are trained to provide care for hypertension and diabetes, in addition to smoking cessation. For smoking cessation, they are given a 1-week training session that focuses on didactics, role play, practicing skills and learning motivational interviewing. A 40-page training book serves as the template for learning. CHWs are also provided a flipbook, and trained in utilizing it for patient education. The CHW finishes training when their overall performance during training is judged by the instructor to be satisfactory. Once fieldwork starts, there is a supervisor whose role is to monitor the work of the CHW using a pre-defined quality checklist to uphold the fidelity of the intervention. These supervisors, in turn, are in regular communication with study investigators (http://fp.amegroups.cn/cms/mhealth.2019.05.04-1.pdf).
mHealth—TxT pro program
Development of the mHealth delivery system
To develop a cost-effective, evidence-based text messaging intervention, we used an existing open source software called RapidPro to develop messaging flows for each stage of behavior change (19). Each messaging flow is triggered by a code word sent by the participant. Given that text messages are delivered solely through the software, with no human mediation, we restricted bidirectional messaging to certain code words that can trigger messaging flows. This will provide a feeling of control to the participant who can decide the type of messages they receive based on their readiness to change. For instance, a previously pre-contemplative participant who is now contemplating quitting can send the words ‘ready for MUKTI’ which triggers the contemplative message flow. This will reduce the passivity of a unidirectional messaging intervention.
Development of the text messages
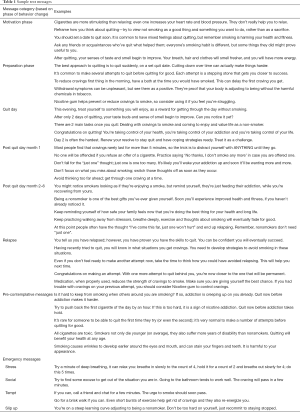
The content of the messages was obtained from a large bank of messages that had been previously used in an Australian trial (20). These messages were modified to suit the needs of our target population. The modified messages were reviewed by multiple study personnel, sample patients and community health workers until all team members agreed upon the final text messages. Over the course of one month, a random sample of these modified messages were delivered through the RapidPro software to evaluate the feasibility of the intervention. Qualitative feedback was obtained from the participants and further changes to the content was made accordingly. Once finalized, the messages were divided into different ‘sets’, to be delivered at different ‘phases’ of the intervention. In addition, through random messages interspersed in the program, participants will be informed of the methods of opting out of the program or contacting their CHW. All messages were translated to Bengali (Dalkhola) and Hindi (Jabalpur). Sample text messages are shown in Table 1.
Full table
Assistance messages
Participants who abstain from smoking, after their preset ‘quit date’, will be provided with a list of code words which can be used to trigger a set of pre-defined assistance messages. Code words such as ‘TEMPT’ or ‘STRESS’ can be sent by the participant which will activate a flow of messages to be delivered over a span of 15 minutes. The goal of these messages is to provide 24-hour assistance and distract an individual from the instant urge to smoke a cigarette/bidi.
Delivery of messages
The intervention software is built to deliver messages randomly between 10AM and 9PM on each day. The number of messages delivered in each week is pre-determined, with a greater number of messages delivered over the weekend. The participants will incur no charge to receive messages. However, depending on their network plan, they may incur a one-time small charge on sending the prespecified code word to trigger the intervention flow.
Recruitment
Recruitment will be done by the CHW, who will visit every household and interview all potential participants listed in the voter’s list. She will also determine the participant’s stage of behavior change by asking them if they would be willing to quit smoking in the next 30 days. Those who answer “Yes or maybe” to this question will be deemed to be contemplative by the CHW and those who respond “No” will be considered pre-contemplative.
Intervention
Rationale and structure
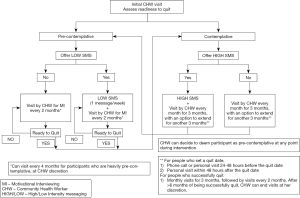
The intervention is tailored to each participant’s stage of behavior change, determined by the CHW at the first home visit. All participants will have the option of choosing between an intervention involving regular text messages combined with scheduled CHW house visits or CHW visits only with no text messages. The intervention structure is described in Figure 2.
Precontemplation
The CHW, at the time of enrollment, will explain the features and benefits of the TxT Pro program and offers this intervention to all participants who are pre-contemplative about quitting smoking. Regardless of their interest in the TxT Pro program, each pre-contemplative participant will receive a CHW visit every 2 months. The CHW will utilize techniques of health education (using a flipbook) and motivational interviewing to communicate with participants, with the goal of transitioning them into becoming contemplative about quitting smoking. In the first couple of visits, she will focus on health education, and then transition to a motivational interviewing- based approach in subsequent visits.
Contemplation
All contemplative participants will be offered the TxT Pro program. Similar to the precontemplative group, each participant will be visited by a CHW (at an increased frequency of once a month for three months) regardless of their interest in the TxT Pro program. At the end of 3 months, if the participant hasn’t quit smoking, the CHW can assign him to the precontemplative group or continue the intensive contemplation intervention for another 3 months, based on her judgment.
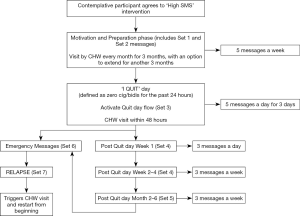
The text messages in this group will differ from those sent to precontemplative participants, and are delivered more frequently (Figure 3). Broadly, these messages are divided into four phases based on a participant’s stage of behavior change, as described below.
Phase I (preparation & motivation)
These messages contain a combination of motivational content, facts regarding smoking and the process of quitting. Participants will be provided with tips to prepare themselves for the process of smoking cessation. Each participant will receive 5 messages a week, delivered at random time points spread throughout the week. During this period, participants will be encouraged to set a quit date and communicate their concerns regarding quitting with the CHW. This phase will continue until they set a quit date, at which point the CHW can transition them to phase II.
Phase II (action)
On the preset ‘quit day’, participants will be expected to have not smoked a single cigarette or bidi for the past 24 hours and inform the CHW by sending the words ‘I QUIT’ to the central number. This message will trigger two integrated events that are focused towards helping the participant stay quit.
First, it will trigger a predefined flow of messages that are designed to encourage the participant to stay quit. During the first 72 hours, they will receive a higher dose (5 messages/day) of text messages to provide additional support. The content of these messages ranges from being congratulatory to those that describe various positive effects of staying away from cigarettes/bidis. Second, it will also trigger a CHW house visit focused towards preventing early relapse. This visit will be within 48 hours of receiving the ‘I QUIT’ message, which is the period of maximum vulnerability for early relapse.
Phase III (post quit)
Three days after quitting, the participant will be transitioned to the post quit phase. It will encompass an early phase (week 1) and a late phase (weeks 2–4). During this phase, the frequency of messages will be gradually decreased but the frequency of CHW house visits will be increased. The dose of messages will be slowly tapered down from 3 messages/day at the beginning to 3 messages/week at the end of the phase. This month-long phase has text messages and CHW visits that encourage sustained abstinence by reminding participants of all the physical, mental and financial benefits of smoking cessation.
This phase will also activate the ‘assistance messages’ which are a pre-defined set of messages that are designed to help participants to stay quit and prevent relapse. These messages are activated by certain code words sent by the participant at a time of need and can be activated at any part of the day.
Phase IV (maintenance)
One month after the quit day, participants who are still quit will be transitioned to the maintenance phase. This phase continues for a duration of 6 months from the quit day and consists of 3 messages a week, coupled with monthly CHW visits to provide brief support and encouragement. These participants continue to have access to ‘assistance messages’ in this phase.
Relapse
At any time after the quit day, if the participant relapses, they will be instructed to send the word ‘RELAPSE’ via a text message. The code word will trigger a set of pre-defined text messages addressing possible solutions for relapse. This will also trigger a CHW visit in the next few days who will examine the various possible causes of relapse and suggest a way forward. If the participant feels they are not ready to quit, the CHW will assign them to the pre-contemplative phase of the intervention.
Access to nicotine replacement therapy
Apart from text messages and CHW home visits, participants will be encouraged to use nicotine replacement therapy to help them during the quitting process. These medications will be made available to them through the CHWs, at market rates. Additionally, participants will also have the option of buying them over the counter. CHWs will be trained about the benefits and risk of NRT, which will also be communicated pictorially to patients through the smoking cessation flipbook used by the CHW.
Control
Screening will be conducted in the same manner as in the intervention group. At the time of screening, all participants will receive brief verbal advice about the hazards of smoking and the benefits of smoking cessation. No further visits or support will be provided for the duration of the intervention.
Outcome evaluation and follow up
All outcomes will be evaluated at the end of 1 year of the intervention. The primary outcome is biochemically verified [by exhaled carbon monoxide (CO) level] abstinence for the past 14 days. CO levels will be checked using a validated breath analyzer by the CHW at her final house visit. Those with a breath CO level of less than 10 ppm will be considered to have quit smoking successfully (21). Pre-specified secondary outcomes will include uptake rates of text messaging and variation in quit rates based on a participant’s education and socio-economic status.
Sample size and statistical analysis
The sample size determinations, or power analysis, account for lack of independence in observations due to the clustered study design, by multiplying the standard sample size calculation by the design effect, {1+[(average cluster size-1)×ICC]}, where the ICC is the intra-cluster correlation coefficient. Sample sizes were calculated using a two-sided test, α=0.05 and power of 80%. Sixteen clusters will be chosen across 2 sites—12 in Dalkhola and 4 in Jabalpur. We fixed the number of clusters for each group at 8 based on our financial and technical capabilities. We used conservative estimates of the population prevalence of smoking to determine the number of participants required.
We used an ICC estimate of 0.04 based on a previous community-based smoking cessation study (22). CHW interventions in non-communicable diseases have usually had an attrition rate of 20–25%, due to the community-based nature of the intervention and broad inclusion criteria (23). We chose a slightly higher attrition rate of 30%, to ensure we had adequate power. We chose a minimum detection rate of 3.9% based on a prior study using CHWs that found a difference of 2.1% between the intervention and control groups (9). We adjusted that upwards to 3.9% due to the longer duration and more intensive nature of the intervention in our study. We calculated that to achieve a minimum detectable difference of 3.9% in quit rates amongst intervention and control group, assuming a conservative ICC of 0.04 and 30% attrition, we would need an average of 35 participants in each of the 16 clusters. We therefore aim to enroll a total of 560 participants. The primary analysis of the study will be done by an intention to treat approach.
Data management
All data collected by the CHWs will be entered at the field office into REDCAP (Research Electronic Data Capture), a secure web application for managing online databases. Only de-identified data will be accessed at University Hospitals of Cleveland, which is the coordinating center for the trial. The central phone, through which the software platform for text messaging is operational, will be securely stored in the field office at the 2 sites. Information from the phone will be accessible only to research personnel at each site.
Limitations
Our study has a few limitations. Participants who smoke but don’t have access to a cell phone are excluded from the trial. This may limit the eventual reach of our intervention. Second, we limited our intervention to smoked tobacco, though a substantial part of the population uses smokeless tobacco. If our intervention is successful, it could be suitably adapted to study its efficacy for quitting smokeless tobacco. Third, the control group participants will only receive brief advice at the start of the trial and no text messages. This places a limitation on differentiating the effect of the content of messages from the delivery of messages itself.
Discussion
Our study has multiple strengths. To our knowledge, this is the first intervention to combine a CHW based approach to smoking cessation with text messaging. Second, the feasibility and efficacy of delivering motivational interviewing through CHWs for any health-related outcomes have not been examined previously. If successful, motivational interviewing through CHWs could be used for other health imperatives. Third, our intervention includes participants who are pre-contemplative about quitting smoking. Historically, this has been a challenging population to treat. If successful, our intervention would create a holistic framework to help all people who smoke, and not just those willing to quit. Fourth, our trial design is real world and pragmatic, and would be generalizable and scalable to a large population of smokers- if successful.
Going forward, low- and middle-income countries are in dire need of models to provide smoking cessation support to an ever-increasing population of individuals who smoke. Our intervention provides a framework that covers all aspects of smoking cessation, and if successful, can be integrated into national CHW programs.
Acknowledgments
The authors would like to thank Prof. Ron Borland who is the Nigel Gray Distinguished Fellow in Cancer Prevention at VicHealth Centre for Tobacco Control, Cancer Council Victoria, Melbourne, Vic., Australia for his guidance during the development of the text messages used in this trial.
Funding: The work was supported by the Society for Enhanced Health and Access to Treatments (SEHAT), which is a charitable non-profit organization based in West Bengal, India. The first author, AK, is the co-founder of SEHAT. Dr Hejjaji is supported by the National Heart, Lung, And Blood Institute of the National Institutes of Health under Award Number T32HL110837.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study protocol received approval from the institutional review board of University Hospitals Cleveland Medical Center, USA and Society for the Promotion of Ethical Clinical Trials (SPECT), an independent review committee in New Delhi, India. All trial participants provided written informed consent and the trial was registered in the clinicaltrials.gov database (registration number- NCT03495622).
References
- Jha P, Jacob B, Gajalakshmi V, et al. A Nationally Representative Case–Control Study of Smoking and Death in India. N Engl J Med 2008;358:1137-47. [Crossref] [PubMed]
- Lim SS, Vos T, Flaxman AD, et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012;380:2224-60. [Crossref] [PubMed]
- Reddy KS, Perry CL, Stigler MH, et al. Differences in tobacco use among young people in urban India by sex, socioeconomic status, age, and school grade: assessment of baseline survey data. Lancet 2006;367:589-94. [Crossref] [PubMed]
- Jha P. Avoidable global cancer deaths and total deaths from smoking. Nat Rev Cancer 2009;9:655-64. [Crossref] [PubMed]
- World Health Organization. WHO report on the global tobacco epidemic, 2017: monitoring tobacco use and prevention policies: executive summary. UCSF: Center for Tobacco Control Research and Education. Available online: https://escholarship.org/uc/item/8nw5p0zt
- Kaur J, Jain DC. Tobacco Control Policies in India: Implementation and Challenges. Indian J Public Health 2011;55:220. [Crossref] [PubMed]
- Lewin S, Munabi-Babigumira S, Glenton C, et al. Lay health workers in primary and community health care for maternal and child health and the management of infectious diseases. Cochrane Database Syst Rev 2010.CD004015. [PubMed]
- Khetan AK, Purushothaman R, Chami T, et al. The Effectiveness of Community Health Workers in CVD Prevention in LMIC. Glob Heart 2017;12:233-243.e6. [Crossref] [PubMed]
- Sarkar BK, West R, Arora M, et al. Effectiveness of a brief community outreach tobacco cessation intervention in India: a cluster-randomised controlled trial (the BABEX Trial). Thorax 2017;72:167-73. [Crossref] [PubMed]
- Khetan A, Purushothaman R, Zullo M, et al. Rationale and design of a cluster-randomized controlled trial to evaluate the effects of a community health worker based program for cardiovascular risk factor control in India. Am Heart J 2017;185:161-72. [Crossref] [PubMed]
- Khetan AK, Zullo MZ, Gupta RG, et al. P3162 Effect of a community health worker based approach to integrated cardiovascular risk factor control in india: a cluster randomized, controlled, parallel-group trial (SEHAT). Eur Heart J 2018;39:ehy563-P3162. [Crossref]
- Scott-Sheldon LAJ, Lantini R, Jennings EG, et al. Text Messaging-Based Interventions for Smoking Cessation: A Systematic Review and Meta-Analysis. JMIR Mhealth Uhealth 2016;4:e49. [Crossref] [PubMed]
- Free C, Knight R, Robertson S, et al. Smoking cessation support delivered via mobile phone text messaging (txt2stop): a single-blind, randomised trial. Lancet 2011;378:49-55. [Crossref] [PubMed]
- Prochaska JO, Velicer WF. The transtheoretical model of health behavior change. Am J Health Promot 1997;12:38-48. [Crossref] [PubMed]
- Lindson-Hawley N, Thompson TP, Begh R. Motivational interviewing for smoking cessation. Cochrane Database Syst Rev 2015.CD006936. [PubMed]
- Weijer C, Grimshaw JM, Eccles MP, et al. The Ottawa Statement on the Ethical Design and Conduct of Cluster Randomized Trials. PLoS Med 2012;9:e1001346. [Crossref] [PubMed]
- Ranking of Districts by Literacy Rate in 2001 and 2011 [Internet]. Government of India; [cited 2015 Nov 11]. Available online: http://censusindia.gov.in/2011-prov-results/prov_data_products_wb.html
- Khetan A, Patel T, Hejjaji V, et al. Role development of community health workers for cardiovascular disease prevention in India. Eval Program Plann 2018;67:177-83. [Crossref] [PubMed]
- Welcome to the RapidPro Community [Internet]. [cited 2018 Dec 7]. Available online: https://community.rapidpro.io/
- Borland R, Balmford J, Benda P. Population-level effects of automated smoking cessation help programs: a randomized controlled trial. Addiction 2013;108:618-28. [Crossref] [PubMed]
- Brose LS, Tombor I, Shahab L, et al. The effect of reducing the threshold for carbon monoxide validation of smoking abstinence-Evidence from the English Stop Smoking Services. Addict Behav 2013;38:2529-31. [Crossref] [PubMed]
- Siddiqi K, Khan A, Ahmad M, et al. Action to stop smoking in suspected tuberculosis (ASSIST) in Pakistan: a cluster randomized, controlled trial. Ann Intern Med 2013;158:667-75. [Crossref] [PubMed]
- Jafar TH, Hatcher J, Poulter N, et al. Community-based interventions to promote blood pressure control in a developing country: a cluster randomized trial. Ann Intern Med 2009;151:593-601. [Crossref] [PubMed]
Cite this article as: Khetan A, Hejjaji V, Hughes J, Gupta P, Barbhaya D, Madan Mohan SK, Josephson RA. Rationale and design of a study to test the effectiveness of a combined community health worker and text messaging-based intervention for smoking cessation in India (Project MUKTI). mHealth 2019;5:15.