Physical activity monitors can be successfully implemented to assess perioperative activity in urologic surgery
Introduction
Wearable technologies and physical activity monitors (PAMs) are an increasing portion of the mobile health (mHealth) technology sector (1). Consumers utilize these technologies for their own wellness tracking, and their applicability in a clinical setting is a rapidly expanding area of interest (2). Early evidence suggests that PAM enhance perioperative fitness (3-6), facilitate discharge planning (7,8), and improve long-term outcomes (9-13).
In a recent survey of urology patients, 82% were willing to use PAM as part of their care and 20% had already adopted this technology (14). While this data suggests that there is an increasing prevalence of such technology, the benefit and indications for PAM in urology remains unknown. Several early studies have suggested improvements in perioperative exercise capacity and physical activity following radical prostatectomy (12,13). Nevertheless, there is a need to define a standardized model wherein these technologies can be effectively implemented while we refine our understanding of their applicability in both inpatient and ambulatory settings. A standardized model would help address existing clinical concerns regarding patient compliance, data/information privacy, and streamline data retrieval (15). It would also address patient concerns regarding data privacy and ease of use which ultimately influence successful adoption of PAM (14).
Currently, tools to assess patient perspectives of PAM technology and activity data in the perioperative period are lacking. Therefore, we sought to create a reproducible model for PAM implementation in the perioperative period that would accurately assess biophysical parameters and patient acceptance. This pilot study was performed in men with localized prostate cancer undergoing robot-assisted radical prostatectomy (RARP) at a single institution. As a secondary outcome, we sought to characterize alterations in post-prostatectomy activity parameters including daily steps, minutes slept, and nighttime awakenings in the early postoperative period.
Methods
Patient selection
After institutional review board approval (Mayo Clinic IRB 15-006885), men undergoing RARP by three surgeons were preoperatively screened for participation in this pilot study from February to October 2016. Men were excluded if they underwent postoperative catheter removal at another institution or lacked a mobile device capable of implementing relevant PAM applications. Men undergoing RARP were chosen as the study population due to standardization of the surgical procedure, inpatient care, and postoperative follow-up.
Study design
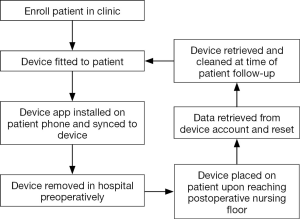
Following written informed consent, Fitbit® Charge HRTM (San Francisco, CA, USA) devices were temporarily provided to patients who met study criteria. Numeric identification was used for all Fitbit® online accounts to maintain patient confidentiality and patient details were only available to study staff. The accompanying Fitbit® mobile application was downloaded on the patient’s device during the preoperative visit. In the event that patients owned a Fitbit® device, they were permitted to use this and granted permission for study staff to temporarily retrieve their activity data around the time of surgery. All pertinent information was maintained in a secure database accessible by only designated study personnel. The account data was cleared from patient devices at the time of device retrieval. Figure 1 depicts the general study overview.
In order to accurately capture activity data, patients were encouraged to wear the device continuously until the day of catheter removal. On the day of surgery, the device was placed on the patient in the postoperative recovery area. The device was retrieved by study staff at the time of urinary catheter removal which was generally one week following surgery.
Given the lack of validated questionnaires assessing patient perspectives with PAM, we developed the Mobile Physical Activity Monitor Questionnaire (MPAMQ). This was designed to assess pre- and postoperative perceptions associated with PAM use (supplementary). Creation of this questionnaire was developed utilizing the validated technology acceptance model (TAM) construct with questions specifically designed to assess opinions of PAM (16-20).
Data analysis
Differences between pre- and postoperative characteristics were assessed. Perioperative biometric data including total daily steps, and sleep quality was obtained from the devices and presented as medians and interquartile ranges and analyzed with Wilcoxon rank-sum test. Patient compliance was calculated by dividing the number of days with daytime (steps) or nighttime (sleep) data by the number of days with the device in the pre- or postoperative setting. Step data was further stratified by non-obese (BMI <30) or obese (BMI ≥30) and non-elderly (age˂65) or elderly (age ≥65 years old). Pre- and postoperative survey responses were tabulated. The frequency of responses of “agree” or “strongly agree” for positive questions and “disagree” or “strongly disagree” for negative questions is presented. All data was analyzed with JMP (SAS Institute, Minneapolis, MN, USA).
Results
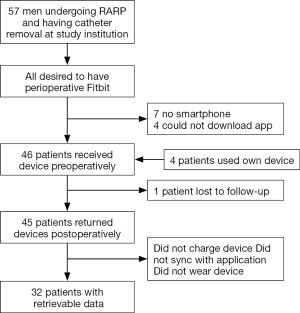
Of the 57 patients screened, 46 (81%) were included in this pilot study (Figure 2). Of the 11 patients excluded, seven (64%) did not have a device capable of downloading the mobile app and four (36%) could not download the mobile app at the time of enrollment. No patients refused participation at time of enrollment. Perioperative patient PAM data was available in 32 patients (70%). In the first 20 patients, the data retrieval rate was 55%, compared to 85% in the last 26 patients. Four patients used their own device and one device was not returned.
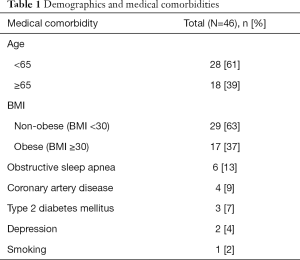
Median age was 63 (IQR, 59–66) years old and BMI was 29 (IQR, 26.6–31.7). Medical comorbidities are listed in Table 1. Only two patients were not dismissed on postoperative day one, but both were dismissed on day two. There were no Clavien-Dindo grade ≥2 complications (21), and the only noted postoperative complication was an asymptomatic umbilical hernia in one patient that has not required intervention.
Full table
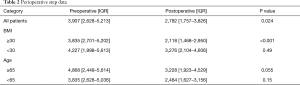
The median duration of PAM use preoperatively was one (range: 1–28) days and seven (range: 6–15) days postoperatively. Daytime compliance was 96% and nighttime compliance was 75%. Perioperative step data can be seen in Table 2. The median postoperative steps were 2,782 (IQR, 1,757–3,826), which was significantly lower than preoperative daily steps [3,907 (IQR, 2,628–5,213); P=0.024]. There was no statistically significant difference in postoperative compared to preoperative minutes slept [358 (IQR, 317–414) vs. 395 (IQR, 306–465); P=0.33] or nighttime awakenings [2.7 (IQR, 1.9–8.9) vs. 3.3 (IQR, 1.0–9.0); P=0.37].
Full table
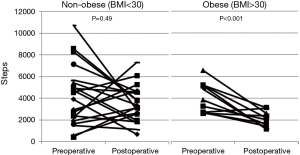
In the non-obese (BMI <30) group (n=20), there was no statistically significant difference in median postoperative compared to preoperative steps [3,276 (IQR, 2,104–4,606) vs. 4,227 (IQR, 1,998–5,613), P=0.49]. However in obese (BMI ≥30) patients (n=12) with PAM data, median postoperative steps were significantly lower than preoperative daily steps [2,118 (IQR, 1,468–2,950) vs. 3,835 (IQR, 2,701–5,202), P<0.001] (Figure 3). Obese patients took 35% fewer steps postoperatively compared with non-obese patients (P=0.017). There was no statistically significant difference in minutes slept (P=0.40) or nighttime awakenings (P=0.47) between groups postoperatively.
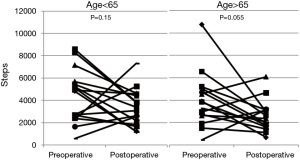
In older (age ≥65) men (n=16), the difference between postoperative steps (median 3,228; IQR, 1,923–4,529) and preoperative steps (median 4,868; IQR, 2,449–5,614) approached statistical significance (P=0.055) (Figure 4). There was no significant difference in younger patients (n=16) postoperative compared to preoperative step counts [2,484 (IQR, 1,627–3,156) vs. 3,835 (IQR, 2,628–5,036); P=0.15]. Postoperative steps (P=0.21), minutes slept (P=0.60) or nighttime awakenings (P=0.91) were similar between younger and older patients.
MPAMQ survey data was available in 45 (98%) patients preoperatively and 42 (91%) postoperatively. Preoperatively, 73% felt comfortable using mobile applications and 82% felt there would be a medical benefit to PAM. Only 4% reported that it would be difficult to learn how to use PAM. Before surgery, only 53% felt PAM would increase their level of activity, this increased to 83% after surgery. Postoperatively at follow-up, 95% of patients were satisfied with using PAM, 48% would pay out of pocket for this technology in the perioperative setting, and 55% planned on using PAM in the future. Most patients (76%) reported that PAM increased physical fitness awareness and 62% agreed that PAM increased their activity from baseline.
Discussion
This is the first pilot study in urology to explore a standardized process for the implementation of PAM use in the perioperative period. Using a widely available, compatible, and adopted PAM platform (Fitbit®), we were able to achieve an 81% accrual rate. Patients reported a medical benefit from perioperative PAM usage and 95% expressed satisfaction with this PAM model. With a high level of compliance (average of 96% during daytime), we demonstrate that RARP reduces ambulation by 29%, and obese patients ambulated 35% less than non-obese patients postoperatively. Minutes asleep and nighttime awakenings, despite patients having an indwelling catheter, were not significantly different. This pilot study suggests that PAM use in the perioperative setting is a viable tool to monitor and enhance patient recovery.
Patient perception and perceived value of PAM is very important to further adoption. Preoperatively, patients were receptive to PAM use, as 82% perceived a benefit to its use. Although studies investigating patient perceptions of PAM are lacking, this is consistent with previously reported data evaluating urology specific populations (14). Almost half of patients would pay out of pocket to use PAM and over half planned on using PAM in the future. Accordingly, ease of use, confidentiality, and low cost have been shown to be associated with willingness to utilize PAM (14), and we designed our model to address many of these issues (Figure 1).
Data processing and real-time interpretation from PAM devices are existing challenges that have been described (15,22). While many PAM devices can provide continuous or near continuous data acquisition, how to interpret and integrate this immense data inflow into existing care pathways remains challenging. For the purpose of this pilot, we controlled for this by averaging daily step counts, sleep and nighttime awakening data from the preoperative and postoperative period. The time to extract and merge this data to a larger database was between 5–7 minutes per patient, which would allow for monitoring of patient activity potentially in real-time to provide early intervention.
Patient compliance is another aspect of this model that demonstrates its utility. Daytime compliance was 95% and nighttime compliance was 74%, which is similar to a PAM study in orthopedic surgery (8). Compliance is higher in the daytime than nighttime due to some individuals not wearing and/or charging the device at night. Other wireless technology studies have had lower rates of compliance (11); in this study automated PAM syncing with the mobile application enabled patients to simply wear the device and charge it periodically. Reducing patient burden and ease of use is an important factor to increase PAM adoption (14). As PAM evolve to become more user-friendly and battery life improves, we would anticipate increasing patient interest and compliance, even among the most challenging populations.
As the pilot study progressed, we were able to refine and streamline our process. Accordingly, data retrieval significantly increased throughout the study period as we gained experience with the model. One unique way we were able to increase data capture was to individually engrave and identify the device and link them with an account. This allowed for more efficient data tracking in devices that were otherwise identical. Patients were also educated on charging the device and syncing them, as these were initially found to be barriers to data capture.
Studies with PAM also require an engaged staff that are familiar with mobile applications, smartphone platforms, and can effectively engage and educate patients. Simply installing an app in the office can be complex and time consuming for the urological provider. By engaging ancillary support staff in this process, it allowed dedicated time to install the PAM, educate, and address any current or future concerns that the patient may have. Nevertheless, despite the relatively high accrual and data retrieval rate, it is evident through that further refinement of our model is needed to eliminate these insufficiencies and the burden on ancillary staff and patients. An automated system that would install the device, troubleshoot, and provide patient education would be ideal.
This is the first time step data collected with PAM has been reported comparing pre- and postoperative periods in urology. We demonstrated a reduction in postoperative steps after prostatectomy, especially in obese patients. While there were no complications associated with a reduction in activity levels, we can extrapolate that that this data may be useful for improved counseling regarding postoperative ambulation knowing that obese patients are increasingly vulnerable to deep venous thrombosis (DVT) (23). Further studies should be done in other pelvic surgery procedures with higher rates of DVT, like cystectomy (24), where this information could be vitally impactful in reducing postoperative morbidity.
The utility of this model can be extrapolated to multiple areas of perioperative care. There is an increased interest in prehabilitation before urologic surgery, including a prospective trial (3,25). In addition, there have been multiple post-prostatectomy rehabilitation programs that have demonstrated successful improvement in functional (continence after prostatectomy), physical, and emotional outcomes (10,12,13). Other studies have been performed outside of urology with similar results (4,5). These models involved structured exercise in either supervised or unsupervised settings. In a systematic review of exercise programs in patients with prostate cancer in various stages of treatment, supervised programs provided superior results to unsupervised programs (10). There are also discordance patient reported outcomes and functional outcomes reported when comparing patient questionnaires to PAM data (26). This provides an opportunity to utilize remote PAM monitoring in unsupervised exercise programs to gain similar benefits to those with direct supervision.
There are limitations to this pilot study. It is a single center experience with all male patients undergoing prostatectomy for prostate cancer. Given the nature of prostate cancer, this is an older male population and thus patient reported perceptions and compliance may not be widely generalizable. Studies are needed to confirm reproducibility of the model outside of the post-prostatectomy setting and to validate the MPAMQ. Pre- and postoperative times with PAM provide further insight to longer trends in activity in the perioperative period. This patient cohort did not have any postoperative complications, albeit the rate of complication rate of RARP is low. A larger study may help define a critical amount of activity in which complications are reduced, as step data has already been proven effective in predicting discharge planning for other major surgeries (7,8). Although data was captured in real time, it was retrospectively retrieved. The process of continuous data monitoring, identifying concerning findings, and clinical intervention is important and needs to be addressed. Indeed, as these devices continue to integrate into healthcare delivery models, remote and real-time ambulatory monitoring has the potential to augment and redefine future surgical care.
Conclusions
The use of perioperative PAM is well accepted with a high level of patient compliance and satisfaction. When effectively implemented, these emerging mHealth technologies and PAM may provide important health-related information for both patients and surgeons in the perioperative period. Important trends in activity, especially in the obese patient, can be used to design further studies and counsel target high-risk patient populations regarding postoperative ambulation. Future research is needed to implement PAM in other urologic and non-urologic surgeries, study activity patterns in perioperative setting, and determine clinical indications where PAM will be of greatest utility.
Mobile Physical Activity Monitor Questionnaire (MPAMQ)
Introduction
The Mayo Clinic Center for Innovation and Department of Urology are prospectively collecting information regarding the usefulness of remote physical activity monitors (PAMs) among patients undergoing surgery. As a Mayo Clinic patient who is currently undergoing treatment by the Department of Urology, you have been selected to participate. Your participation in this survey is voluntary and greatly appreciated. All responses are anonymous and no identifying information will be recorded.
Definition
Mobile PAM (i.e., Fitbit, Nike Fuel, Jawbone, etc.) is a wearable device which continuously monitors and records the user’s daily activity (steps and distance traveled), energy expenditure, dietary intake, sleep patterns, and heart rate. The user can monitor activity via mobile application (i.e., smartphone or tablet) or through a desktop application (i.e., personal computer).
Demographic
1. Do you have a working computer, laptop, netbook, tablet, iPad, or video-enabled smartphone?
Yes No
2. Do you have broadband internet at home?
Yes No
3. Do you have previous experience with mobile physical activity monitoring devices (i.e., Fitbit, Nike Fuel, Jawbone, etc.)?
Yes No
4. I currently use a wearable device for monitoring of activity or health?
Yes No
5. If yes, which one(s)? (Fitbit, Apple Watch, Google Glass, Jawbone, Life Alert, pedometer, heart rate monitor, other) ________________
6. Which best describes your state of health?
1) My health makes it impossible for me to engage in most activities
2) My health makes it impossible for me to engage in some activities
3) My health makes it difficult for me to engage in some activities
4) I am able go about my daily activities with minimal difficulty
5) I am fully active without restriction
7. Can I climb 2 flights of stairs without stopping to rest?
1) Yes with no difficulty
2) Yes, with difficulty
3) No, can’t do at all
4) Don’t know
8. Do I get short of breath with current activity levels?
Yes No
Self-efficacy
9. I frequently use the internet
1) Strongly disagree
2) Disagree
3) Neutral
4) Agree
5) Strongly agree
10. Taking an active role in my own health care is an important factor in determining my health and ability to function
1) Strongly disagree
2) Disagree
3) Neutral
4) Agree
5) Strongly agree
11. I am proficient at using mobile device applications
1) Strongly disagree
2) Disagree
3) Neutral
4) Agree
5) Strongly agree
12. I believe that my level of physical fitness will impact the outcome of my treatment
1) Strongly disagree
2) Disagree
3) Neutral
4) Agree
5) Strongly agree
13. I am proficient at using computer software applications
1) Strongly disagree
2) Disagree
3) Neutral
4) Agree
5) Strongly agree
14. I am proficient at using mobile physical activity monitors to track my physical activity
1) Strongly disagree
2) Disagree
3) Neutral
4) Agree
5) Strongly agree
15. I am confident that I would be able to effectively use a mobile physical activity monitor based on my level of computer expertise
1) Strongly disagree
2) Disagree
3) Neutral
4) Agree
5) Strongly agree
16. I have rich experiences with mobile physical activity monitors
1) Strongly disagree
2) Disagree
3) Neutral
4) Agree
5) Strongly agree
17. I am able to use the mobile physical activity device properly
1) Strongly disagree
2) Disagree
3) Neutral
4) Agree
5) Strongly agree
Technical support and training
18. I will need technical support to use a mobile physical activity monitoring device
1) Strongly disagree
2) Disagree
3) Neutral
4) Agree
5) Strongly agree
19. Without technical support, a mobile physical activity monitoring device will not be useful
1) Strongly disagree
2) Disagree
3) Neutral
4) Agree
5) Strongly agree
Attitude
20. I feel that monitoring my health by using a wearable device will improve my health
1) Strongly disagree
2) Disagree
3) Neutral
4) Agree
5) Strongly agree
21. Using the mobile physical activity monitor during my cancer treatment is a good idea
1) Strongly disagree
2) Disagree
3) Neutral
4) Agree
5) Strongly agree
22. Using the mobile physical activity monitor would be unpleasant
1) Strongly disagree
2) Disagree
3) Neutral
4) Agree
5) Strongly agree
23. I dislike the idea of using a mobile physical activity monitor program
1) Strongly disagree
2) Disagree
3) Neutral
4) Agree
5) Strongly agree
24. I believe that a wearable device would improve communication with my health care provider?
1) Strongly agree
2) Agree
3) Neutral
4) Disagree
5) Strongly disagree
Perceived usefulness
25. I believe there is a medical benefit from using a wearable physical activity monitoring device
1) Strongly disagree
2) Disagree
3) Neutral
4) Agree
5) Strongly agree
26. Using a mobile physical activity monitor will increase my level of physical activity
1) Strongly disagree
2) Disagree
3) Neutral
4) Agree
5) Strongly agree
27. Using a mobile physical activity monitor will be of benefit to my treatment and recovery
1) Strongly disagree
2) Disagree
3) Neutral
4) Agree
5) Strongly agree
28. I think that the use of a mobile physical activity monitor would enhance the effectiveness of my treatment
1) Strongly disagree
2) Disagree
3) Neutral
4) Agree
5) Strongly agree
29. I think that monitoring my physical activity would make my treatment more effective
1) Strongly disagree
2) Disagree
3) Neutral
4) Agree
5) Strongly agree
30. The following are potential benefits of wearable physical activity monitoring technology (select all that apply)
1) Independence of Living
2) Convenience for health monitoring
3) Feedback related to individual health needs and goals
4) Increased safety
5) Improvement in function
6) Ease in communication with physician
7) Increased access to media/social media
8) None
Perceived ease of use
31. It will be difficult to learn how to use the mobile physical activity monitor
1) Strongly disagree
2) Disagree
3) Neutral
4) Agree
5) Strongly agree
32. I would find it easy to get the mobile physical activity monitor to do what I want it to do
1) Strongly disagree
2) Disagree
3) Neutral
4) Agree
5) Strongly agree
33. It will not be easy to become skillful in the use of the mobile physical activity monitor
1) Strongly disagree
2) Disagree
3) Neutral
4) Agree
5) Strongly agree
34. I think it will be easy to operate the mobile physical activity monitor
1) Strongly disagree
2) Disagree
3) Neutral
4) Agree
5) Strongly agree
Behavioral intent to use
35. I would be willing to use a wearable device to monitor my physical activity
1) Strongly disagree
2) Disagree
3) Neutral
4) Agree
5) Strongly agree
36. What kind of device would you most prefer to use?
1) Fitbit
2) Google Glass
3) Apple Watch
4) Jawbone
5) Pedometer
6) Heart rate monitor
7) Other/don’t know ____________
37. I have the intention to use the mobile physical activity monitor in my treatment
1) Strongly disagree
2) Disagree
3) Neutral
4) Agree
5) Strongly agree
38. I intend to use the mobile physical activity monitor as much as possible in my treatment
1) Strongly disagree
2) Disagree
3) Neutral
4) Agree
5) Strongly agree
39. If possible, I intend not to use the mobile physical activity monitor in my treatment
1) Strongly disagree
2) Disagree
3) Neutral
4) Agree
5) Strongly agree
40. I would be willing to monitor the following with a wearable device? (Select all that apply)
1) Monitor my daily activity log (pedometer)
2) Monitor my blood pressure
3) Monitor heart rate
4) Monitor my diet intake/weight
5) Social/media communication
6) Access to emergency response system
7) Monitor post procedure status
Costs
41. I would pay out of pocket costs to use a mobile physical activity monitor
1) Strongly disagree
2) Disagree
3) Neutral
4) Agree
5) Strongly agree
42. I would only use a mobile physical activity monitor if it was covered by my insurance
1) Strongly disagree
2) Disagree
3) Neutral
4) Agree
5) Strongly agree
Post-use questions
43. Using a mobile physical activity monitor has increased my level of activity above baseline
1) Strongly disagree
2) Disagree
3) Neutral
4) Agree
5) Strongly agree
44. Using a mobile physical activity monitor has increased my awareness of physical fitness
1) Strongly disagree
2) Disagree
3) Neutral
4) Agree
5) Strongly agree
45. Using a mobile physical activity monitor has improved my health
1) Strongly disagree
2) Disagree
3) Neutral
4) Agree
5) Strongly agree
46. Using a mobile physical activity monitor has improved my quality of life
1) Strongly disagree
2) Disagree
3) Neutral
4) Agree
5) Strongly agree
47. I am satisfied with my experience using a mobile physical activity monitor
1) Strongly disagree
2) Disagree
3) Neutral
4) Agree
5) Strongly agree
48. I intend to use a mobile physical activity monitor after completion of my treatment
1) Strongly disagree
2) Disagree
3) Neutral
4) Agree
5) Strongly agree
49. Do you have any additional input regarding your experience? ___________
Acknowledgements
This work was supported by the Mayo Clinic Center for Innovation.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by institutional review board (Mayo Clinic IRB 15-006885) and written informed consent was obtained from all patients.
References
- Fox S, Duggan M. Mobile Health 2012.
- Appelboom G, Yang AH, Christophe BR, et al. The promise of wearable activity sensors to define patient recovery. J Clin Neurosci 2014;21:1089-93. [Crossref] [PubMed]
- Carli F, Awasthi R, Gillis C, et al. Optimizing a frail elderly patient for radical cystectomy with a prehabilitation program. Can Urol Assoc J 2014;8:E884-E7. [Crossref] [PubMed]
- Hayashi K, Hirashiki A, Kodama A, et al. Impact of preoperative regular physical activity on postoperative course after open abdominal aortic aneurysm surgery. Heart Vessels 2016;31:578-83. [Crossref] [PubMed]
- Li C, Carli F, Lee L, et al. Impact of a trimodal prehabilitation program on functional recovery after colorectal cancer surgery: a pilot study. Surg Endosc 2013;27:1072-82. [Crossref] [PubMed]
- Gillis C, Li C, Lee L, et al. Prehabilitation versus Rehabilitation: A Randomized Control Trial in Patients Undergoing Colorectal Resection for Cancer. Anesthesiology 2014;121:937-47. [Crossref] [PubMed]
- Cook DJ, Thompson JE, Prinsen SK, et al. Functional Recovery in the Elderly After Major Surgery: Assessment of Mobility Recovery Using Wireless Technology. Ann Thorac Surg 2013;96:1057-61. [Crossref] [PubMed]
- Toogood PA, Abdel MP, Spear JA, et al. The monitoring of activity at home after total hip arthroplasty. Bone Joint J 2016;98-b:1450-4.
- Appelboom G, Taylor BE, Bruce E, et al. Mobile Phone-Connected Wearable Motion Sensors to Assess Postoperative Mobilization. JMIR Mhealth Uhealth 2015;3. [Crossref] [PubMed]
- Baumann FT, Zopf EM, Bloch W. Clinical exercise interventions in prostate cancer patients--a systematic review of randomized controlled trials. Support Care Cancer 2012;20:221-33. [Crossref] [PubMed]
- Dawes AJ, Reardon S, Chen VL, et al. Wireless Technology to Track Surgical Patients after Discharge: A Pilot Study. Am Surg 2015;81:1061-6. [PubMed]
- Park SW, Kim TN, Nam JK, et al. Recovery of Overall Exercise Ability, Quality of Life, and Continence After 12-Week Combined Exercise Intervention in Elderly Patients Who Underwent Radical Prostatectomy: A Randomized Controlled Study. Urology 2012;80:299-305. [Crossref] [PubMed]
- Zopf EM, Bloch W, Machtens S, et al. Effects of a 15-Month Supervised Exercise Program on Physical and Psychological Outcomes in Prostate Cancer Patients Following Prostatectomy. Integr Cancer Ther 2015;14:409-18. [Crossref] [PubMed]
- Nehra AK, Gettman MT, Rivera ME, et al. Patients are Willing to Utilize Wearable Devices for Their Care: A Survey of Perceptions and Acceptance of Wearable Technology for Health Monitoring in a Urological Patient Population. Urology Practice 2017;4:508-14. [Crossref]
- Majmudar MD, Colucci LA, Landman AB. The quantified patient of the future: Opportunities and challenges. Healthcare 2015;3:153-6. [Crossref] [PubMed]
- Davis FD. Perceived Usefulness, Perceived Ease of Use, and User Acceptance of Information Technology. MIS Quarterly 1989;13:319-40. [Crossref]
- Gagnon MP, Orruno E, Asua J, et al. Using a modified technology acceptance model to evaluate healthcare professionals' adoption of a new telemonitoring system. Telemed J E Health 2012;18:54-9. [Crossref] [PubMed]
- Jeon E, Park HA. Factors affecting acceptance of smartphone application for management of obesity. Healthc Inform Res 2015;21:74-82. [Crossref] [PubMed]
- Cranen K, Veld RH, Ijzerman M, et al. Change of patients' perceptions of telemedicine after brief use. Telemed J E Health 2011;17:530-5. [Crossref] [PubMed]
- Rho MJ, Choi IY, Lee J. Predictive factors of telemedicine service acceptance and behavioral intention of physicians. Int J Med Inform 2014;83:559-71. [Crossref] [PubMed]
- Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205-13. [Crossref] [PubMed]
- Shelgikar AV, Anderson PF, Stephens MR. Sleep Tracking, Wearable Technology, and Opportunities for Research and Clinical Care. Chest 2016;150:732-43. [Crossref] [PubMed]
- Anderson FA Jr, Spencer FA. Risk factors for venous thromboembolism. Circulation 2003;107:I9-16. [Crossref] [PubMed]
- White RH, Zhou H, Romano PS. Incidence of symptomatic venous thromboembolism after different elective or urgent surgical procedures. Thromb Haemost 2003;90:446-55. [PubMed]
- Jensen BT, Petersen AK, Jensen JB, et al. Efficacy of a multiprofessional rehabilitation programme in radical cystectomy pathways: a prospective randomized controlled trial. Scand J Urol 2015;49:133-41. [Crossref] [PubMed]
- Mobbs RJ, Phan K, Maharaj M, et al. Physical Activity Measured with Accelerometer and Self-Rated Disability in Lumbar Spine Surgery: A Prospective Study. Global Spine J 2016;6:459-64. [Crossref] [PubMed]
Cite this article as: Agarwal DK, Viers BR, Rivera ME, Nienow DA, Frank I, Tollefson MK, Gettman MT. Physical activity monitors can be successfully implemented to assess perioperative activity in urologic surgery. mHealth 2018;4:43.