A technology ecosystem for chronic pain: promises, challenges, and future research
Being a common, mostly unpredictable, highly personalized manifestation, chronic pain is thought to be one of the most promising conditions in which the use of digital health technologies may boost patient’s engagement and coping. In fact, pain treatment, besides pharmacotherapy, involves non-pharmacotherapeutic management, which may include physical and psychological interventions. Self-monitoring, real-time assessment, timely feedback, treatment options information, social and community support, and connection with a multidisciplinary care team are among the features that can mostly help chronic pain sufferers to enhance disease and comorbidities management and, ultimately, improve their quality of life (1).
However, more evidence is still needed to understand how these technologies should be best designed and implemented to tackle chronic pain.
In their paper “Correlation of digital health use and chronic pain coping strategies”, Ranney and colleagues (2) explored the use of digital health technologies among patients with chronic pain participating in “Patients Like Me”, an online initiative devoted to promote connection and experience sharing between patients with chronic conditions. Administering a cross-sectional survey to almost 600 patients, they showed that chronic pain patients commonly use digital health technologies and that the use allows an improvement on coping mechanisms (2). In particular, the authors concluded that, even though online resources are the most addressed, there is an increasing trend towards the adoption of social and mHealth personalized technologies (apps and social media) that are used by 1/3 of the survey participants.
Their results further support the perceived beneficiary effect of personal health technologies for the self-management of chronic conditions (3), that, together with the disrupting increase of mobile connections (that has now passed the number of individuals in several Countries, including Europe and US) depicts a positive scenario fostering a paradigm change in chronic pain care. Personalized interventions, more tailored to individual needs, implementing a holistic approach in a bio-psychological framework can be developed allowing better access to care and ensuring comprehensive treatment plans for the majority of the population. Inclusion of the majority of the population is indeed one of the most compelling advantages brought by mobile technologies (4), and recent reports (http://www.slideshare.net/wearesocialsg/digital-in-2016) show that the “technology gap” among generations and gender is rapidly decreasing.
Despite these appealing considerations, there are caveats to be taken into account, especially when patients rely on the use of mHealth apps (5-8): security and privacy issues, quality of the contents, communications with healthcare professionals and data exchange with electronic health records are the most critical in the present scenario.
mHealth data regulation is still under definition since the technologies available on the market are outside the classical healthcare institutional boundaries, and are not covered by the Health Insurance Portability and Accountability Act of 1996 (HIPAA) that regulates only “covered entities” (9). Individuals using mHealth apps may therefore have a limited understanding on the extent to which the data they use, save, and share in such applications are protected by law. Also, the level of security standards adopted by the organizations promoting mHealth apps may not be disclosed, and patients may be not aware of the cybersecurity threats they may face. Finally, it is not clear the potential secondary use of the huge amount of health data collected by mHealth apps and devices: who is the owner of those data? Are they anonymously stored? Are they aggregated or individual? All these questions are still unanswered and represent one important drawback for a full adoption of personal mHealth technologies to support all chronic conditions, and not only pain.
A second point regards the quality of the apps proposed to the patient/consumer from the well-known app stores. A review conducted in 2014 underlined the lack of correspondence between the solutions for pain management described in the scientific literature and those available in the app stores (6). Of the 47 papers published on 34 apps in scientific databases, none was available in the app stores, and of the 283 pain-related apps found in the five stores searched, none was published in a scientific journal. The split between scientific evidence and commercially-available products has been confirmed in a recent review on smartphone applications for pain management (1): the authors downloaded and tested 195 apps, and proposed a checklist for quality assessment. They found that, even though chronic pain requires an holistic and evidence-based approach including bio-psychosocial components (e.g., cognitive behavioral therapies, CBT), few of the apps evaluated included proper source references, used standardized clinical scales, or recognized interventions, and that only one can be considered as appropriate for pain management. Additional apps for pain management reported in literature are listed in Table 1. All these findings pose serious concerns about the possible harmfulness of mHealth apps available for download, often for free, on major app stores, and suggest that research institutions are non-connected with the developers marketing the Apps. There is not a shared framework for app evaluation, and, while researchers are aware of the possible risks posed by apps developed with little or no scientific evidence, patients may either not understand the possible harms or not know where to find trustful suggestions. Unfortunately, the problem of app quality is still a challenge for eHealth research: some solutions have been proposed, ranging from HON-code like codes of conducts (19), to auto-certification synopsis for developers (20), to pictorial schemas highlighting risks and benefits from multiple viewpoints (21,22).
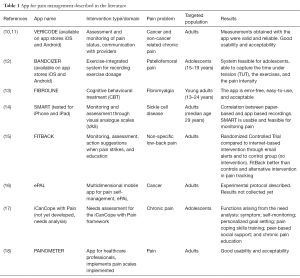
Full table
Finally, the lack of communication between the patients and the care team, as well as the low inclusion of patient-generated data to electronic health records (EHRs) may limit the efficacy of the intervention (7,10,23). At present, despite the general agreement on the need of including mHealth app in the “healthIT ecosystem” thus allowing the patient to be an active part of her/his care process, patient-generated data using mHealth apps remain outside the sets of trustful information that are included in health information systems and EHRs (24). Standards-based architectures were proposed to implement the bi-directional communication between mHealth apps and EHRs (7,23) ensuring a meaningful and secure data exchange, a proper technological and semantic interoperability, patient’s education, and evidence-based practice. Even though these architectures are still to be validated in everyday practice, they provide a guideline to design effective systems for chronic pain patients (Figure 1), able to overcome the major present limitations.
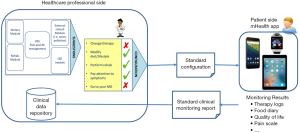
The integrated system depicted in Figure 1 is composed of two main modules, one dedicated to healthcare professionals and the other one dedicated to patients (mHealth app running on different personal devices) that implement a decision-support system (DSS) providing tailored suggestions and alerts, supporting self-monitoring, disease control, and decision-making. In this proposed architecture, the DSS is semi-automatic: suggestions are meant to be validated by healthcare professionals before being delivered to the patients, in order to avoid potential risks and to design an optimal personalized lifestyle/therapeutic plan. In this way, the architecture allows decreasing the risks of erroneous/unwanted suggestions thus ensuring better control of the suggested care pathway. The DSS inputs should come from both the mHealth app, as monitoring data, relevant behavioral and environmental data, etc., and from the patient’s EHR, where the clinical assessments are stored, in order to depict a more comprehensive assessment of the patient’s status. Hence, the DSS reasoning is based on both patient-generated measures (mediated by the mHealth app) and clinical measures (available on the EHR). The communication interface between the EHR and the mHealth app should be implemented using standard structured documents such as the modified personal healthcare monitoring (PHMR) template used in (23). In this way, the mHealth app and the EHR are fully independent and the same app could dialogue with different EHR systems, and does not require the development of an ad-hoc EHR.
The only constraint is that the healthcare professional platform should also contain the DSS engine, so that the mHealth solution should act only as a tool for data collection, suggestions/alerts notification, and patient’s education. The EHR system should also be responsible of data storing and management, and, to ensure data protection, should allow the sharing of only de-identified data. No relevant or personal data should be stored on the mHealth app, which is considered as a non-secure environment. On the patient’s side, the mHealth app allows patient’s monitoring through ad-hoc questionnaires, clinical scales, diaries, as well as proactive support for patient’s therapy, communication with the care team, education, and social interaction. The same solution could be used to administer CBTs. The mHealth app may take advantage of existing systems/applications aimed to collect relevant data that can be integrated to obtain the various inputs that the DSS would require. In addition, existing devices could be used to collect data related to environmental information, through sensors (e.g., noise).
In conclusion, the promising scenario for personalized, patient-centered, and holistic pain management boosted by the widespread use of health digital technologies still requires efforts in terms of security, quality, and interoperability, to be fully exploited. Even though further research is needed, standards-based architectures relying on a tight interaction between healthcare teams and patients may be the way to overcome the present limitations.
Acknowledgements
S Marceglia’s research is supported by the grant GR-2011-02352807 from the Italian Ministry of Health.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Portelli P, Eldred C. A quality review of smartphone applications for the management of pain. Br J Pain 2016;10:135-40. [Crossref] [PubMed]
- Ranney ML, Duarte C, Baird J, et al. Correlation of digital health use and chronic pain coping strategies. mHealth 2016;2:35. [Crossref]
- Frisbee KL. Variations in the Use of mHealth Tools: The VA Mobile Health Study. JMIR Mhealth Uhealth 2016;4:e89. [Crossref] [PubMed]
- Müller AM, Alley S, Schoeppe S, et al. The effectiveness of e-& mHealth interventions to promote physical activity and healthy diets in developing countries: A systematic review. Int J Behav Nutr Phys Act 2016;13:109. [Crossref] [PubMed]
- Marceglia S, Bonacina S, Zaccaria V, et al. How might the iPad change healthcare? J R Soc Med 2012;105:233-41. [Crossref] [PubMed]
- de la Vega R, Miró J. mHealth: a strategic field without a solid scientific soul. a systematic review of pain-related apps. PLoS One 2014;9:e101312. [Crossref] [PubMed]
- Marceglia S, Fontelo P, Ackerman MJ. Transforming consumer health informatics: connecting CHI applications to the health-IT ecosystem. J Am Med Inform Assoc 2015;22:e210-2. [Crossref] [PubMed]
- Albrecht UV, von Jan U. mHealth Apps and Their Risks - Taking Stock. Stud Health Technol Inform 2016;226:225-8. [PubMed]
- Examining Oversight of the Privacy & Security of Health Data Collected by Entities Not Regulated by HIPAA. Available online: [cited 2017 Jan 16].https://www.healthit.gov/sites/default/files/non-covered_entities_report_june_17_2016.pdf
- Jamison RN, Jurcik DC, Edwards RR, et al. A Pilot Comparison of a Smartphone App with or without 2-Way Messaging Among Chronic Pain Patients: Who Benefits from a Pain App? Clin J Pain 2016. [Epub ahead of print]. [Crossref] [PubMed]
- Jamison RN, Mei A, Ross EL. Longitudinal trial of a smartphone pain application for chronic pain patients: Predictors of compliance and satisfaction. J Telemed Telecare 2016. [Epub ahead of print]. [Crossref] [PubMed]
- Rathleff MS, Bandholm T, McGirr KA, et al. New exercise-integrated technology can monitor the dosage and quality of exercise performed against an elastic resistance band by adolescents with patellofemoral pain: an observational study. J Physiother 2016;62:159-63. [Crossref] [PubMed]
- de la Vega R, Roset R, Galán S, et al. Fibroline: A mobile app for improving the quality of life of young people with fibromyalgia. J Health Psychol 2016. [Epub ahead of print]. [Crossref] [PubMed]
- Jonassaint CR, Shah N, Jonassaint J, et al. Usability and Feasibility of an mHealth Intervention for Monitoring and Managing Pain Symptoms in Sickle Cell Disease: The Sickle Cell Disease Mobile Application to Record Symptoms via Technology (SMART). Hemoglobin 2015;39:162-8. [Crossref] [PubMed]
- Irvine AB, Russell H, Manocchia M, et al. Mobile-Web app to self-manage low back pain: randomized controlled trial. J Med Internet Res 2015;17:e1. [Crossref] [PubMed]
- Agboola S, Kamdar M, Flanagan C, et al. Pain management in cancer patients using a mobile app: study design of a randomized controlled trial. JMIR Res Protoc 2014;3:e76. [Crossref] [PubMed]
- Stinson JN, Lalloo C, Harris L, et al. iCanCope with Pain™: User-centred design of a web- and mobile-based self-management program for youth with chronic pain based on identified health care needs. Pain Res Manag 2014;19:257-65. [Crossref] [PubMed]
- de la Vega R, Roset R, Castarlenas E, et al. Development and testing of painometer: a smartphone app to assess pain intensity. J Pain 2014;15:1001-7. [Crossref] [PubMed]
- Lewis TL. A systematic self-certification model for mobile medical apps. J Med Internet Res 2013;15:e89. [Crossref] [PubMed]
- Albrecht UV. Transparency of health-apps for trust and decision making. J Med Internet Res 2013;15:e277. [Crossref] [PubMed]
- Bonacina S, Marceglia S, Pinciroli F. A pictorial schema for a comprehensive user-oriented identification of medical Apps. Methods Inf Med 2014;53:208-24. [Crossref] [PubMed]
- Basilico A, Marceglia S, Bonacina S, et al. Advising patients on selecting trustful apps for diabetes self-care. Comput Biol Med 2016;71:86-96. [Crossref] [PubMed]
- Marceglia S, Fontelo P, Rossi E, et al. A Standards-Based Architecture Proposal for Integrating Patient mHealth Apps to Electronic Health Record Systems. Appl Clin Inform 2015;6:488-505. [Crossref] [PubMed]
- Conceptualizing a Data Infrastructure for the Capture, Use, and Sharing of Patient-Generated Health Data in Care Delivery and Research through 2024. Available online: [cited 2017 Jan 16].https://www.healthit.gov/sites/default/files/Draft_White_Paper_PGHD_Policy_Framework.pdf
Cite this article as: Marceglia S, Conti C. A technology ecosystem for chronic pain: promises, challenges, and future research. mHealth 2017;3:6.


