Leveraging telemedicine in gastroenterology and hepatology: a narrative review
Introduction
The field of medicine is in a rapidly changing state, with rising costs and evolving challenges in healthcare needs, and as such new solutions are continually in development. Telemedicine is one such solution and refers to the two-way exchange of communication through audio and/or video, thus allowing physicians to provide medical care remotely. Telehealth is a mode of healthcare that encompasses sharing and communicating health data through technological devices across distances to facilitate virtual healthcare. Telemedicine is a part of the broader term “telehealth”, and is defined as the direct communication between a provider and their patient across distances, where medical information is communicated virtually (1).
Over the years, especially throughout the coronavirus disease 2019 (COVID-19) pandemic, telemedicine has played a pivotal role in counteracting the changing demands and culture of medicine. While telemedicine has been widely used in certain specialties, its usage in gastroenterology (GI) remains sparse. In this review, we provide an overview of telemedicine, its history, and its applications in regards to managing GI and hepatology care. We present this article in accordance with the Narrative Review reporting checklist (available at https://mhealth.amegroups.com/article/view/10.21037/mhealth-23-27/rc).
Methods
This narrative review was written based on searches from electronic databases, including PubMed and Google Scholar. Specifically, we searched for articles that included the words telehealth, telemedicine, GI, hepatology, artificial intelligence (AI), tele-education, and health equity. There was no specific time frame used during the literature search. Our search strategy is described in Table 1.
Table 1
| Items | Specification |
|---|---|
| Date of search | August 1st, 2023 |
| Databases and other sources searched | PubMed Central and Google Scholar |
| Search terms used | “Gastroenterology AND telemedicine” |
| “Telehealth” | |
| “Hepatology AND telemedicine” | |
| “Telemedicine AND education” | |
| “Telemedicine AND artificial intelligence” | |
| “Telemedicine AND health equity OR health disparities” | |
| Timeframe | January 1977 to July 2023 |
| Inclusion criteria | Articles written in English language |
| Selection process | One author (VA) conducted selection |
Historical overview of telemedicine and definitions
Through a series of technological innovations, telemedicine has evolved and adapted to become a plausible method of healthcare delivery to patients with various medical needs. In the 19th century, the development of telegraphs and Morse code allowed for the transmission of medical information across distances. In fact, initial reports of telemedicine explored the idea of using telephones as tools to auscultate lung and heart sounds (2). During the mid-1900s, other forms of communication emerged (Figure 1). The radio and the telephone allowed for the sharing of medical information in addition to facilitating communication between providers. In the past two decades, computers and the internet have enhanced access to large clinical data and allowed for the increasing access to telecommunication (3). To this day, advancements in medicine and technology continue to guide the evolution of telemedicine.
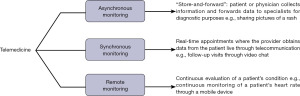
Currently, the three types of telemedicine are synchronous, asynchronous, and remote monitoring (Figure 2) (4). Synchronous telemedicine consists of the real-time health care delivery through tele-medicine appointments, where healthcare professionals interact with the patients and provide clinical guidance through virtual visits. Asynchronous telemedicine, also known as “store-and-forward”, consists of the collection of patient information and transmission to a healthcare provider for review. Lastly, the remote monitoring type of telemedicine refers to the continuous assessment of a patient’s condition involving medical diagnosis, history, and/or new symptoms, all monitored virtually through different devices. Given its flexibility with different formats, patients can access healthcare through their mobile devices, interact virtually with healthcare professionals, and implement health monitoring devices for managing diseases. In addition to providing benefits for patient care, telemedicine can also be leveraged to provide remote education for residents and physicians through tele-education.
Although the concept of telemedicine has been around for many years, confusion still exists between the terms “telemedicine” and “telehealth”. In particular, telemedicine is commonly confused with the term “telehealth”, which is an umbrella term for the former. The lack of clear definitions results in some organizations, such as the National Cancer Institute, using the words interchangeably (5). Both telemedicine and telehealth consist of the prefix “tele”, which translates to “at a distance” in Greek (6). Telehealth encompasses a wider variety of health services and can be utilized by various health professionals (e.g., nurses, social workers, pharmacists, physicians). Telemedicine, on the other hand, is usually utilized by physicians for the remote delivery of healthcare (7). Although the definitions of telehealth and telemedicine seem similar, their usage in the medical literature varies. For example, a bibliometric analysis of 11,644 articles found that the term “telemedicine” (n=8,028) was more commonly used in research titles and abstracts compared to telehealth (n=1,679) (8). Furthermore, advancements in telehealth have resulted in the birth of new terms such as mHealth and eHealth (Table 2). More specifically, mHealth refers to the utilization of mobile devices for health monitoring, while eHealth involves the use of information and communications technologies in health care (9,10). As technology continues to advance and intersect with medicine, it would be no surprise if we notice an increase in novel terms and modifications to preexisting definitions.
Table 2
| Telehealth and its variations | Definition |
|---|---|
| Telehealth | Telehealth is a mode of healthcare delivery by a medical team that involves sharing data through technology |
| Telemedicine | Telemedicine is the sharing of medical information/advice by a physician in one location, and the patient in a different location |
| Telecare | Telecare involves using technological platforms that serve for patients to independently monitor their health and to connect with their caretakers/support system |
| eHealth | Electronic (e)-Health refers to the global way of thinking about healthcare, public health, and business through technology such as the Internet and satellite communication to improve local and global healthcare |
| mHealth | Mobile (m)-Health refers to the use of mobile devices and other wearable technologies to detect biological changes while monitoring patients and relaying this data to health management groups, such as clinics and providers |
COVID-19 and its impact on GI care
With the onset of the COVID-19 pandemic, telemedicine became a more viable and acceptable option for receiving and delivering medical care. In addition, studies have shown a significant increase in the number of publications regarding telehealth, further indicating the expanding use of this platform (11). For example, in 2019, less than 1% of Medicare primary care visits in the United States were through telehealth; alternatively, in April 2020, this number grew to 46% before dropping to approximately 15% toward the end of 2022 (12,13).
The usage of telemedicine in GI has historically been low relative to other internal medicine specialties. Prior to the COVID-19 pandemic, only 7.9% of gastroenterologists reported using telemedicine in practice, ranking it second lowest across internal medicine specialties (14). Its low usage has changed dramatically since the pandemic, wherein 57% of gastroenterologists reported using telemedicine at least once (15). Before the pandemic, telemedicine in GI was primarily used to manage patients with inflammatory bowel disease (IBD) (16-18) and chronic liver diseases (19,20). Mandated lockdown orders during the pandemic resulted in the need to implement safe and effective practices for the management of various gastroenterologic and hepatologic disorders. Consequently, GI providers relied considerably on technology to deliver safe, remote medical care.
Telemedicine applications in GI and hepatology
Despite its sparse presence in GI and hepatology, telemedicine offers many benefits for patients and physicians. The COVID-19 pandemic has accelerated the use of telemedicine while also providing physicians with more information regarding the benefits and pitfalls of incorporating telemedicine in GI and hepatology care. In the following section, we discuss the applications of telemedicine in managing various gastroenterologic and hepatologic diseases, and its importance in physician education.
IBD
IBD, which encompasses Crohn’s disease and ulcerative colitis (UC), is characterized by chronic inflammation of the GI tract and affects over 3 million Americans (21). In addition to the decrease in quality of life (QOL), patients with IBD incur a significantly higher annual direct cost of care than non-IBD patients ($22,987 vs. $6,956 per year) and more than twice the out-of-pocket healthcare costs (22). Moreover, treating IBD requires multiple strategies, including achieving tight disease control, adherence to treatment regimens, and monitoring side effects (23). As such, stakeholders in the healthcare system strive to implement treatment plans to alleviate the financial burden associated with IBD care while streamlining and improving patient care.
Several studies have demonstrated the benefits of telemedicine over conventional office visits for patients with IBD. In a study at the Royal Brisbane and Women’s Hospital in Australia, out of the first 153 patients surveyed, 94% rated their telehealth experience as “excellent” or “very good”, with 99% of patients deciding to continue their treatment via telehealth (24). Another study conducted in the Netherlands aimed to explore differences between telemedicine and conventional office follow-ups for pediatric patients with IBD. In this multicenter randomized trial, researchers demonstrated that the telehealth cohort reported a slight increase in QOL, albeit not statistically significant, and a mean annual cost saving of 89 euros, which increased to 360 euros for those compliant throughout the entirety of the study (25). The cost-effectiveness of telehealth is noted in other studies across various medical specialties (26-28).
Given the often unpredictable nature of IBD due, for instance, to disease flares (i.e., relapses), remote monitoring (i.e., telemonitoring) can be leveraged to monitor disease status from a distance. Telemonitoring platforms such as “HealthPROMISE”, a cloud-based app developed for patients with IBD, allow users to track their symptoms, medications, QOL scores, and quality of care (QOC) scores via questionnaires (29). The information documented by patients is available to providers in real time, allowing providers to track their patients’ most current health status. A randomized control trial conducted at Mount Sinai Medical Center investigated the impact of HealthPROMISE in improving QOC and QOL among 320 patients with IBD (30). During a median follow-up of 495 days (±135), the patients assigned to the HealthPROMISE cohort reported a significantly higher QOC compared to the control cohort (28% vs. 9%; P<0.01) along with improvements in QOL. Another study found that patients who utilized HealthPROMISE for one year reported a significant decrease in ER visits/hospitalizations compared to the year before when the app was not utilized (25% vs. 3%; P=0.03) (31). Furthermore, patients reported increased understanding of their disease etiology upon using the mobile app, highlighting yet another benefit of telemedicine: increasing patient education.
Another telemonitoring platform called “Constant-Care”, developed in 2009 for patients with UC, allows users to document relapse and remission events, and complete disease activity questionnaires (32). By utilizing questionnaires such as the Short Inflammatory Bowel Disease Questionnaire and the Simple Colitis Clinical Activity Index, Constant-Care can project disease activity as a traffic light with three colors: red, yellow, or green. Red light indicates highly active UC, yellow is moderately active, and green is inactive. In the event of a relapse, the program is able to recommend treatment with 4 grams of 5-aminosalicylic acid (5-ASA) and recommends a maintenance dosage when patients enter remission (33). In a randomized trial conducted in Denmark and Ireland, patients who utilized Constant-Care had a shorter duration of relapse in comparison to the control group (median, 18 vs. 77 days), in addition to an increase in treatment adherence (34). Furthermore, at the time of relapse, 100% of patients using Constant-Care began the recommended treatment with 5-ASA compared to only 10% of patients in the control cohort (P<0.0001). The success of Constant-Care as a telemonitoring platform is noted across other studies as well (35,36).
Celiac disease (CD)
CD is a chronic autoimmune condition induced by gluten ingestion, affecting 1% of the world population (37). Currently, the only treatment for CD involves a lifelong diet of gluten-free products. However, given the strict diet regimen for CD, many patients struggle with adhering to treatment plans resulting in a decrease in QOL. Implementing diet and lifestyle changes are essential for improving CD symptoms, preventing intestinal damage, and improving psychological symptoms.
Mobile applications can serve as avenues for monitoring care in patients with CD. MyHealthyGut is the first evidence-based app for patients with CD (38). Notable features of the app include: therapeutic meal plans and recipes, a live Q&A bot, educational content, a virtual health coach, and a report creation platform, allowing users to share information and progress with their physicians in real-time. A 2020 study found that patients who utilized MyHealthyGut were satisfied with the app features, however, they felt that the app would be more helpful to those recently diagnosed with CD (39). Similar to MyHealthyGut, the GlutenFreeDiet application renders users with personalized feedback regarding their dietary profile and other personalized parameters (40). Furthermore, results from a randomized control clinical trial in Iran showed that CD patients who utilized a mobile application for CD care reported significantly lower indigestion scores (P<0.001) compared to the control group, highlighting the role of mobile applications for improving GI related symptoms (41). Another study from the Netherlands found that CD patients who utilized online consultations reported improvements in QOL and a mean saving of 202 Euros compared to the outpatient consultation group (42). Overall, mobile applications are cost effective modalities that can improve the QOL among CD patients.
In recent years, the development of self-monitoring health technology has allowed patients to monitor adherence to a gluten-free diet. Home-based assays that can detect gluten immunogenic peptides in the urine can inform patients about their inadvertent gluten intake (43). Additionally, questionnaires such as the Celiac Dietary Adherence Test (CDAT) have been successfully utilized by clinicians to monitor adherence to gluten-free diets and can potentially be integrated in a telemedicine setting (44,45).
Hepatitis C virus (HCV)
Globally, the HCV affects 58 million people, with 1.5 million new infections per year, as a result of which there is significant clinical and economic burden (46). Although advancements in antiviral therapies have proven effective in treating HCV, barriers such as distance from providers, treatment adherence, and knowledge of HCV infection can hinder successful treatment (47). For many years, telemedicine has served as an avenue for improving HCV screening and care, especially among people residing in rural areas. One of the most successful interventions for virtual HCV care is the Extension for Community Healthcare Outcomes (ECHO) model. Developed in 2003, ECHO was initially launched to address disparities in HCV care among residents in New Mexico (48). By leveraging digital technology, ECHO serves as a tele-mentoring program that connects rural primary care physicians with HCV specialists via a virtual network. Through ECHO, healthcare providers collaborate in virtual case-based conferences where they share patient medical histories, treatment plans, and lab results. A 2011 study found that patients treated at ECHO sites achieved similar virologic response rates compared to patients treated at an academic health clinic, suggesting the benefits of leveraging technology for patient care collaboration and reaching underserved populations (49). Due to its success, ECHO is now adopted across various medical specialties (50) and is currently used in 194 countries (51).
Telemedicine has also been effective in delivering HCV care to high-risk populations such as people who use drugs (PWUD) and prisoners. PWUDs represent a large majority of HCV infections, yet they are the least likely to seek care due to barriers such as difficulties in linkage of care and low treatment adherence (52). As such, telemedicine can serve as a viable option for overcoming these barriers and guiding treatment among PWUDs. A study conducted at an Italian addiction center found that the implementation of telemedicine resulted in a sustained virologic response (SVR) rate of 98.5%, along with 100% linkage to care among PWUDs (53). Another study at a syringe service program found that telemedicine was successful in achieving a 93.5% SVR in individuals with opioid use disorders (54). Prior to the COVID-19 pandemic, efforts had been made to integrate telemedicine in penitentiaries in order to improve HCV care for incarcerated individuals. For example, a 2019 study performed at a Spanish correctional facility investigated the benefits of telemedicine in an open label program of HCV elimination (55). Prior to the initiation of the telehealth-based program, HCV prevalence among prisoners was 12.4%, which dropped to 0% after the study. In addition to achieving high SVR rates, participating physicians and inmates were highly satisfied with the program. The success of telemedicine in guiding HCV treatment among inmates is noted across other studies before and after the COVID-19 pandemic (56-60).
Liver transplantation
Liver transplant patients (pre- and post-) may live far from their transplant center, and transplant practices are often highly impacted. As such, telemedicine has the potential to serve as an alternative to traditional clinic visits in this vulnerable patient population. A retrospective study at the Richmond Veterans Affairs Medical Center found that patients who utilized telehealth were evaluated significantly faster than patients in the conventional care group (21.7 vs. 79.5 days; P<0.01) and listed on transplant waitlists faster than the control cohort (138.8 vs. 249 days; P<0.01) (61). Furthermore, the introduction of the Specialty Care Access Network-Extension of Community Healthcare Outcomes (SCAN-ECHO), a virtual triage program, across the Veterans Affairs Hospitals has shown to reduce futile transplant evaluations, highlighting the potential of leveraging telehealth in hepatology triaging (62). In another study, Wang et al. developed a virtual frailty screening tool that can be used in a transplant setting for patients with cirrhosis (63). The benefits of utilizing telemedicine are also seen in post-liver transplantation care. Studies show that post-transplantation follow-ups via telemedicine produce fewer commute and waiting times, promote medication adherence, and are satisfactory for patients (64,65). Although these findings are promising, more controlled studies need to be conducted in the future.
Physician education
With telemedicine widely spreading, tele-education has also become an efficient option to teach residents, fellows, students, and even independent practitioners (e.g., gastroenterologists). Tele-education can facilitate better outcomes in managing chronic GI disease given its accessibility and increased opportunity across different healthcare facilities (66). As mentioned earlier, one notable example of tele-education in GI is Project ECHO. After having success as a tele-mentoring platform for rural providers, ECHO has expanded to over 900 partners and millions of users (67). Additionally, in the past decades, there has been an increase in the number of gastrointestinal endoscopy-related teleconferences, such as the Endoscopic Club E-conference (ECE) (68). Conferences such as ECE have the potential to connect physicians from different areas of the globe, enhance collaboration, and promote learning. The development of digital video transport systems has also allowed for the virtual transfer of endoscopic procedures to various academic centers for educational purposes without compromising patient privacy (69,70). In addition to increasing telehealth education among currently practicing physicians, it is essential to implement telehealth education in the medical school curriculum. Consequently, efforts have been made to increase telehealth education among medical students and residents (71). A study from Germany demonstrated that online telehealth modules were successful in familiarizing medical students with telehealth-based systems and telehealth related clinical skills (72). As technology and medicine continue to intersect, tele-education will likely play an essential role for the next generation of healthcare providers.
Integrating telemedicine in GI: where we need to be
Barriers to telemedicine
Despite its many benefits, telemedicine has its concomitant limitations for patients and physicians (Table 3). Patient barriers to virtual care include privacy concerns, limited access to technology and internet, lack of telehealth knowledge, and insurance coverage (73). Although healthcare is a highly regulated industry in the United States, it remains susceptible to cyber-attacks. Interoperability through cloud computing and electronic medical databases have allowed for seamless transfer of patient data; at the same time, however, patient data can be compromised through cyber-attacks and breaches (74). In addition to cybersecurity concerns, lack of robust internet connectivity and bandwidth in remote areas can also impede virtual care for patients. For example, 33% of rural Americans lack access to high-speed internet (>25 Mbps); thus, they are unable to utilize telehealth services (75). Furthermore, lack of technological knowledge can impose significant barriers to telehealth access. A study from the University of Chicago Medical Center found that patients with low eHealth literacy were less likely to use video technology for their telehealth visit (76). Uncertainty around insurance coverage also pose concerns for patients interested in utilizing telehealth. Although reimbursements for telehealth services before the pandemic have been low, insurers have become more flexible during the pandemic due to the changes in healthcare policies (77). To minimize patient barriers to telehealth services it is essential to develop robust computer infrastructures, provide patients with proper equipment and education, and establish permanent policies for telehealth coverage (78).
Table 3
| Advantages | Disadvantages | Where we need to be |
|---|---|---|
| Access to healthcare and health education (e.g., tele-education) for remote populations | Access to telehealth and telemedicine depends on the availability of stable Internet, technological devices, and knowledge of patients | Increasing education about different telemedicine platforms |
| More convenience and time efficient for patients | Risk of an experiencing power outage/unstable Internet connection | Ensuring that patients from different demographics have equitable access to telemedicine services |
| Cost effective | Possibility of data breach/security concerns | Continue to improve cybersecurity and protection of confidential patient information |
| Less exposure to pathogens and allergens | Fragmented insurance policies regarding telemedicine reimbursements | Prompting insurance companies to reimburse a wider range of telemedicine services |
| Effective way to complete follow-up visits, referrals, and long-term patient monitoring | Multiple telemedicine platforms that serve the same function often overwhelming users | Creating robust platforms to streamline patient care |
Similar to patients, providers also face barriers. Provider barriers include licensures, limited technological literacy, and loss of physical assessments (73,79). The difficulty of accessing multistate licensures is a barrier for implementing telemedicine for many physicians (80). Limited technological literacy is another potential barrier for incorporating telemedicine. For example, a study consisting of 136 rural providers from a rural Pennsylvania hospital and its satellite clinics found that 72.6% of physicians reported lack of technological literacy as a barrier for telemedicine usage (79). Another potential barrier is the lack of physical assessments as physical assessments play a vital role in patient care and are limited in virtual care. Solutions to the aforementioned barriers are discussed below.
Achieving health equity in telemedicine
Addressing disparities in telemedicine care and usage is essential for achieving health equity for all. Disparities in telemedicine usage can be attributed to a variety of factors including age, socioeconomic status, technological literacy, and culture (81). For example, a study from the University of California, San Francisco found that Black/African-American and Hispanic patients reported lower telehealth usage in comparison to Non-Hispanic White patients, further illustrating racial disparities in seeking telemedicine care (82). Racial disparities in telemedicine usage can be attributed to the lack of proper internet and equipment also known as the “digital divide” (83). Because telemedicine is highly favored among underserved populations, it is essential to address disparities in technology usage in order to achieve digital health equity (84,85). In addition to racial disparities, elderly patients are also less likely to use telemedicine. Elderly patients report technological literacy, language barriers, and difficulty hearing as barriers for telemedicine use (86,87). To alleviate difficulties in seeking telehealth care among older patients, efforts have been made to make telehealth more feasible for older adults (88,89). For example, the Video Visits for Elders Project (VVEP) consists of four intervention goals designed to increase patient knowledge on telehealth and assist with enabling telehealth platforms on patient devices (89). Patients from low socioeconomic backgrounds also report difficulties accessing telemedicine care (90,91). Implementation of loaner tablet programs can potentially alleviate the disparity in technological access seen among minority and low-income individuals (92). Telemedicine remains a powerful tool in health care delivery, and if utilized correctly, it can reduce the no-show rate in minority populations (93). It is also important to note that disparities in choosing telemedicine modalities (phone call or video call) also exist. A study from Duke University found that elderly and non-Hispanic Black patients with liver disease were more likely to use phone calls over video calls when engaging with their provider (94). Further research needs to be done to determine whether video calls confer advantages over phone calls for patients specifically seeking GI and hepatology care. Overall, in order to achieve health equity in telemedicine, it is essential to understand and dismantle the barriers that minority and elderly populations face in order to prevent further exacerbation of diseases.
In addition to satisfying direct patient needs, uncertainties in health policy and technological security need to be resolved to ensure equitable care. Uncertainty regarding insurance reimbursements can deter minority populations from benefitting from telemedicine services. Recently, Congress passed legislation to extend Medicare flexibilities for telehealth visits through December 31, 2024; however, permanent policies have not been established (95). Without future action from policymakers, millions of Americans are at risk of losing coverage for telehealth visits. Additionally, as discussed earlier, difficulties in obtaining multistate licensure can also prevent physicians from incorporating telemedicine services. A solution to this is adapting legislature that allows for reciprocity for licensure across multiple states (96). By alleviating the burden of licensures, policymakers can play a role in facilitating the implementation of telemedicine among physicians. Lastly, it is essential that telehealth platforms have robust security. Lack of cybersecurity can compromise confidential patient information and result in patient mistrust (74). As we venture into a realm of medicine that is increasingly dependent on technology, telehealth services will become more prominent. As such, it is essential to address barriers to telemedicine in order to achieve health equity for all.
Lack of unified apps
Another limitation to telemedicine usage, as seen for instance in IBD, is the lack of robust telemedicine apps. In hepatology, the Project ECHO platform has proven to be an effective tele-mentoring platform with millions of users. However, telemedicine for IBD remains fragmented. Despite the many benefits of remote monitoring for patients with IBD, the presence of multiple platforms can be daunting for physicians and patients. Efforts to unify and create a robust application to support patients with IBD are needed.
Integrating AI in telemedicine
Currently, AI is a popular topic for many clinicians, including gastroenterologists. While AI has proven useful for detecting polyps during colonoscopies (97), its implications in GI telemedicine are unclear. In the past, researchers have shown that wearable devices that measure heart rate variability can assist with predicating UC flares, highlighting the role of wearable devices for remote patient monitoring (98). Pimentel et al., developed a mobile app that leverages AI to assess stool form (99). Patients diagnosed with diarrhea predominant-IBS were told to take a picture of their stool, which was then analyzed via AI to determine stool characteristics such as consistency, fragmentation, edge fuzziness, and volume. Although the app determined stool characteristics with a high accuracy, more studies need to be conducted to determine its full potential.
Conclusions
With the pressures imposed by the COVID-19 pandemic, rising costs of health care, and the increasing reliance on technology-based health systems, telemedicine has become an ever-important avenue for managing gastroenterologic and hepatologic disorders. Telemedicine can alleviate the economic burden associated of healthcare, save patients time, and serve as an alternative to in-person clinic appointments. However, the full potential of telemedicine in this regard is yet to be determined. More research into the relative strengths and weaknesses of telemedicine coupled with greater real-life experience with telemedicine will increase global understanding in this respect and provide patients and providers a broader and deeper array of options to coordinate care.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://mhealth.amegroups.com/article/view/10.21037/mhealth-23-27/rc
Peer Review File: Available at https://mhealth.amegroups.com/article/view/10.21037/mhealth-23-27/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://mhealth.amegroups.com/article/view/10.21037/mhealth-23-27/coif). JHT serves as an unpaid editorial board member of mHealth from December 2022 to November 2024. The other authors have no conflicts of interest to declare.
Ethical Statement:
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- CDC. Telehealth and Telemedicine: A Research Anthology of Law and Policy Resources 2023. Available online: https://www.cdc.gov/phlp/publications/topic/anthologies/anthologies-telehealth.html
- Aronson SH. The Lancet on the telephone 1876-1975. Med Hist 1977;21:69-87. [Crossref] [PubMed]
- Jagarapu J, Savani RC. A brief history of telemedicine and the evolution of teleneonatology. Semin Perinatol 2021;45:151416. [Crossref] [PubMed]
- Mechanic OJ, Persaud Y, Kimball AB. Telehealth Systems. 2023.
- National Cancer Institute. Dictionary of Cancer Terms 2023. Available online: https://www.cancer.gov/publications/dictionaries/cancer-terms/def/telehealth
- Craig J, Patterson V. Introduction to the practice of telemedicine. J Telemed Telecare 2005;11:3-9. [Crossref] [PubMed]
- Federal Communications Commission. Telehealth, telemedicine, and telecare: What’s What? 2023. Available online: https://www.fcc.gov/general/telehealth-telemedicine-and-telecare-whats-what
- Fatehi F, Wootton R. Telemedicine, telehealth or e-health? A bibliometric analysis of the trends in the use of these terms. J Telemed Telecare 2012;18:460-4. [Crossref] [PubMed]
- Istepanian R, Jovanov E, Zhang YT. Introduction to the special section on M-Health: beyond seamless mobility and global wireless health-care connectivity. IEEE Trans Inf Technol Biomed 2004;8:405-14. [Crossref] [PubMed]
- WHO. eHealth. Available online: https://www.emro.who.int/health-topics/ehealth/
- Doraiswamy S, Abraham A, Mamtani R, et al. Use of Telehealth During the COVID-19 Pandemic: Scoping Review. J Med Internet Res 2020;22:e24087. [Crossref] [PubMed]
- Neri AJ, Whitfield GP, Umeakunne ET, et al. Telehealth and Public Health Practice in the United States-Before, During, and After the COVID-19 Pandemic. J Public Health Manag Pract 2022;28:650-6. [Crossref] [PubMed]
- Services CfMaM. Medicare Telehealth Trends Report 2023. Available online: https://data.cms.gov/sites/default/files/2023-06/Medicare%20Telehealth%20Trends%20Snapshot%2020230523_508.pdf
- Leow AH, Mahadeva S. Telemedicine in routine gastroenterology practice: A boost during the COVID-19 pandemic. JGH Open 2021;5:533-4. [Crossref] [PubMed]
- Patel SY, Mehrotra A, Huskamp HA, et al. Variation In Telemedicine Use And Outpatient Care During The COVID-19 Pandemic In The United States. Health Aff (Millwood) 2021;40:349-58. [Crossref] [PubMed]
- Castro HK, Cross RK, Finkelstein J. Using a Home Automated Telemanagement (HAT) system: experiences and perceptions of patients with inflammatory bowel disease. AMIA Annu Symp Proc 2006;2006:872. [PubMed]
- Ankersen DV, Carlsen K, Marker D, et al. Using eHealth strategies in delivering dietary and other therapies in patients with irritable bowel syndrome and inflammatory bowel disease. J Gastroenterol Hepatol 2017;32:27-31. [Crossref] [PubMed]
- Hansen MR, Ankersen DV, Marker D, et al. Telemedicine applications for monitoring inflammatory bowel disease and irritable bowel syndrome. Ugeskr Laeger. 2020;182:V10190588. [PubMed]
- Stotts MJ, Grischkan JA, Khungar V. Improving cirrhosis care: The potential for telemedicine and mobile health technologies. World J Gastroenterol 2019;25:3849-56. [Crossref] [PubMed]
- Su GL, Glass L, Tapper EB, et al. Virtual Consultations Through the Veterans Administration SCAN-ECHO Project Improves Survival for Veterans With Liver Disease. Hepatology 2018;68:2317-24. [Crossref] [PubMed]
- Dahlhamer JM, Zammitti EP, Ward BW, Wheaton AG, Croft JB. Prevalence of Inflammatory Bowel Disease Among Adults Aged ≥18 Years - United States, 2015. MMWR Morb Mortal Wkly Rep 2016;65:1166-9. [Crossref] [PubMed]
- Park KT, Ehrlich OG, Allen JI, et al. The Cost of Inflammatory Bowel Disease: An Initiative From the Crohn's & Colitis Foundation. Inflamm Bowel Dis 2020;26:1-10. Correction appears in Inflamm Bowel Dis 2020;26:1118.
- Colombel JF, D'haens G, Lee WJ, et al. Outcomes and Strategies to Support a Treat-to-target Approach in Inflammatory Bowel Disease: A Systematic Review. J Crohns Colitis 2020;14:254-66. [Crossref] [PubMed]
- Lim MH, McMahon A, Radford-Smith G. Delivering inflammatory bowel disease care across distance. Intern Med J 2022;52:411-7. [Crossref] [PubMed]
- Heida A, Dijkstra A, Muller Kobold A, et al. Efficacy of Home Telemonitoring versus Conventional Follow-up: A Randomized Controlled Trial among Teenagers with Inflammatory Bowel Disease. J Crohns Colitis 2018;12:432-41. [Crossref] [PubMed]
- de Jong MJ, Boonen A, van der Meulen-de Jong AE, et al. Cost-effectiveness of Telemedicine-directed Specialized vs Standard Care for Patients With Inflammatory Bowel Diseases in a Randomized Trial. Clin Gastroenterol Hepatol 2020;18:1744-52. [Crossref] [PubMed]
- Cuadrado A, Cobo C, Mateo M, et al. Telemedicine efficiently improves access to hepatitis C management to achieve HCV elimination in the penitentiary setting. Int J Drug Policy 2021;88:103031. [Crossref] [PubMed]
- Habashi P, Bouchard S, Nguyen GC. Transforming Access to Specialist Care for Inflammatory Bowel Disease: The PACE Telemedicine Program. J Can Assoc Gastroenterol 2019;2:186-94. [Crossref] [PubMed]
- Atreja A, Khan S, Rogers JD, et al. Impact of the Mobile HealthPROMISE Platform on the Quality of Care and Quality of Life in Patients With Inflammatory Bowel Disease: Study Protocol of a Pragmatic Randomized Controlled Trial. JMIR Res Protoc 2015;4:e23. [Crossref] [PubMed]
- Atreja A, Khan S, Otobo E, et al. P554 Impact of real world home based remote monitoring on quality of care and quality of life in inflammatory bowel disease patients: one year results of pragmatic randomized trial. Journal of Crohn's and Colitis 2017;11:S362-3. [Crossref]
- Zhen J, Marshall JK, Nguyen GC, et al. Impact of Digital Health Monitoring in the Management of Inflammatory Bowel Disease. J Med Syst 2021;45:23. [Crossref] [PubMed]
- Elkjaer M, Burisch J, Avnstrøm S, et al. Development of a Web-based concept for patients with ulcerative colitis and 5-aminosalicylic acid treatment. Eur J Gastroenterol Hepatol 2010;22:695-704. [PubMed]
- Walsh A, Travis S. What's app? Electronic health technology in inflammatory bowel disease. Intest Res 2018;16:366-73. [Crossref] [PubMed]
- Elkjaer M, Shuhaibar M, Burisch J, et al. E-health empowers patients with ulcerative colitis: a randomised controlled trial of the web-guided 'Constant-care' approach. Gut 2010;59:1652-61. [Crossref] [PubMed]
- Pedersen N, Thielsen P, Martinsen L, et al. eHealth: individualization of mesalazine treatment through a self-managed web-based solution in mild-to-moderate ulcerative colitis. Inflamm Bowel Dis 2014;20:2276-85. [Crossref] [PubMed]
- Pedersen N, Elkjaer M, Duricova D, et al. eHealth: individualisation of infliximab treatment and disease course via a self-managed web-based solution in Crohn’s disease. Aliment Pharmacol Ther 2012;36:840-9. [Crossref] [PubMed]
- Caio G, Volta U, Sapone A, et al. Celiac disease: a comprehensive current review. BMC Med 2019;17:142. [Crossref] [PubMed]
- Dowd AJ, Jackson C, Tang KTY, et al. MyHealthyGut: development of a theory-based self-regulatory app to effectively manage celiac disease. Mhealth 2018;4:19. [Crossref] [PubMed]
- Dowd AJ, Warbeck CB, Tang KT, et al. MyHealthyGut: Findings from a pilot randomized controlled trial on adherence to a gluten-free diet and quality of life among adults with celiac disease or gluten intolerance. Digit Health 2020;6:2055207620903627. [Crossref] [PubMed]
- Perez-Junkera G, Vázquez-Polo M, Eizagirre FJ, et al. Application of a Platform for Gluten-Free Diet Evaluation and Dietary Advice: From Theory to Practice. Sensors (Basel) 2022;22:732. [Crossref] [PubMed]
- Nikniaz Z, Namvar ZA, Shirmohammadi M, et al. Smartphone Application for Celiac Patients: Assessing Its Effect on Gastrointestinal Symptoms in a Randomized Controlled Clinical Trial. Int J Telemed Appl 2022;2022:8027532. [Crossref] [PubMed]
- Vriezinga S, Borghorst A, van den Akker-van Marle E, et al. E-Healthcare for Celiac Disease-A Multicenter Randomized Controlled Trial. J Pediatr 2018;195:154-160.e7. [Crossref] [PubMed]
- Moreno ML, Cebolla Á, Muñoz-Suano A, et al. Detection of gluten immunogenic peptides in the urine of patients with coeliac disease reveals transgressions in the gluten-free diet and incomplete mucosal healing. Gut 2017;66:250-7. [Crossref] [PubMed]
- Leffler DA, Dennis M, Edwards George JB, et al. A simple validated gluten-free diet adherence survey for adults with celiac disease. Clin Gastroenterol Hepatol 2009;7:530-6, 536.e1-2.
- Gładyś K, Dardzińska J, Guzek M, et al. Celiac Dietary Adherence Test and Standardized Dietician Evaluation in Assessment of Adherence to a Gluten-Free Diet in Patients with Celiac Disease. Nutrients 2020;12:2300. [Crossref] [PubMed]
- WHO. Hepatitis C. 2022. Available online: https://www.who.int/news-room/fact-sheets/detail/hepatitis-c
- McGowan CE, Fried MW. Barriers to hepatitis C treatment. Liver Int 2012;32:151-6. [Crossref] [PubMed]
- Arora S, Geppert CM, Kalishman S, et al. Academic health center management of chronic diseases through knowledge networks: Project ECHO. Acad Med 2007;82:154-60. [Crossref] [PubMed]
- Arora S, Thornton K, Murata G, et al. Outcomes of treatment for hepatitis C virus infection by primary care providers. N Engl J Med 2011;364:2199-207. [Crossref] [PubMed]
- Kenny C, Priyadarshini A. "Mind the Gap" - An overview of the role of the Extensions Community Healthcare Outcomes (ECHO) model in enhancing value in health care delivery. AIMS Public Health 2023;10:94-104. [Crossref] [PubMed]
- Sciences UH. Project ECHO. 2023. Available online: https://hsc.unm.edu/echo/partner-portal/data-marketplace/
- Nevola R, Rosato V, Conturso V, et al. Can Telemedicine Optimize the HCV Care Cascade in People Who Use Drugs? Features of an Innovative Decentralization Model and Comparison with Other Micro-Elimination Strategies. Biology (Basel) 2022;11:805. [Crossref] [PubMed]
- Rosato V, Nevola R, Conturso V, et al. Telemedicine Improves HCV Elimination among Italian People Who Use Drugs: An Innovative Therapeutic Model to Increase the Adherence to Treatment into Addiction Care Centers Evaluated before and during the COVID-19 Pandemic. Biology (Basel) 2022;11:800. [Crossref] [PubMed]
- Sivakumar A, Madden L, DiDomizio E, et al. Treatment of Hepatitis C virus among people who inject drugs at a syringe service program during the COVID-19 response: The potential role of telehealth, medications for opioid use disorder and minimal demands on patients. Int J Drug Policy 2022;101:103570. [Crossref] [PubMed]
- Jiménez Galán G, Alia Alia C, Vegue González M, et al. The contribution of telemedicine to hepatitis C elimination in a correctional facility. Rev Esp Enferm Dig 2019;111:550-5. [Crossref] [PubMed]
- Halder A, Li VG, Sebastian M, et al. Use of telehealth to increase treatment access for prisoners with chronic hepatitis C. Intern Med J 2021;51:1344-7. [Crossref] [PubMed]
- Morey S, Hamoodi A, Jones D, et al. Increased diagnosis and treatment of hepatitis C in prison by universal offer of testing and use of telemedicine. J Viral Hepat 2019;26:101-8. [Crossref] [PubMed]
- Richter V, Goldstein L, Cohen DL, et al. The effect of direct-acting antiviral regimens and telemedicine on the treatment of inmates with hepatitis C virus infection in Israeli prisons. Sci Prog 2022;105:368504221105173. [Crossref] [PubMed]
- Sterling RK, Cherian R, Lewis S, et al. Treatment of HCV in the Department of Corrections in the Era of Oral Medications. J Correct Health Care 2018;24:127-36. [Crossref] [PubMed]
- Talal AH, Andrews P, Mcleod A, et al. Integrated, Co-located, Telemedicine-based Treatment Approaches for Hepatitis C Virus Management in Opioid Use Disorder Patients on Methadone. Clin Infect Dis 2019;69:323-31. [Crossref] [PubMed]
- John BV, Love E, Dahman B, et al. Use of Telehealth Expedites Evaluation and Listing of Patients Referred for Liver Transplantation. Clin Gastroenterol Hepatol 2020;18:1822-1830.e4. [Crossref] [PubMed]
- Konjeti VR, Heuman D, Bajaj JS, et al. Telehealth-Based Evaluation Identifies Patients Who Are Not Candidates for Liver Transplantation. Clin Gastroenterol Hepatol 2019;17:207-209.e1. [Crossref] [PubMed]
- Wang M, Shui AM, Barry F, et al. The tele-liver frailty index (TeLeFI): development of a novel frailty tool in patients with cirrhosis via telemedicine. Am J Transplant 2023;23:966-75. [Crossref] [PubMed]
- Le LB, Rahal HK, Viramontes MR, et al. Patient Satisfaction and Healthcare Utilization Using Telemedicine in Liver Transplant Recipients. Dig Dis Sci 2019;64:1150-7. [Crossref] [PubMed]
- Tian M, Wang B, Xue Z, et al. Telemedicine for Follow-up Management of Patients After Liver Transplantation: Cohort Study. JMIR Med Inform 2021;9:e27175. [Crossref] [PubMed]
- Siegel CA. Transforming Gastroenterology Care With Telemedicine. Gastroenterology 2017;152:958-63. [Crossref] [PubMed]
- Project ECHO 2022 Annual Report 2022. Available online: https://projectechoannualreport.unm.edu.
- Ho SH, Rerknimitr R, Kudo K, et al. Telemedicine for gastrointestinal endoscopy: The Endoscopic Club E-conference in the Asia Pacific Region. Endosc Int Open 2017;5:E244-52. [Crossref] [PubMed]
- Shimizu S, Itaba S, Yada S, et al. Significance of telemedicine for video image transmission of endoscopic retrograde cholangiopancreatography and endoscopic ultrasonography procedures. J Hepatobiliary Pancreat Sci 2011;18:366-74. [Crossref] [PubMed]
- Kaltenbach T, Muto M, Soetikno R, et al. Teleteaching endoscopy: the feasibility of real-time, uncompressed video transmission by using advanced-network technologies. Gastrointest Endosc 2009;70:1013-7. [Crossref] [PubMed]
- Bajra R, Frazier W, Graves L, et al. Feasibility and Acceptability of a US National Telemedicine Curriculum for Medical Students and Residents: Multi-institutional Cross-sectional Study. JMIR Med Educ 2023;9:e43190. [Crossref] [PubMed]
- Vogt L, Schmidt M, Follmann A, et al. Telemedicine in medical education: An example of a digital preparatory course for the clinical traineeship - a pre-post comparison. GMS J Med Educ 2022;39:Doc46. [PubMed]
- Perisetti A, Goyal H. Successful Distancing: Telemedicine in Gastroenterology and Hepatology During the COVID-19 Pandemic. Dig Dis Sci 2021;66:945-53. [Crossref] [PubMed]
- Wasserman L, Wasserman Y. Hospital cybersecurity risks and gaps: Review (for the non-cyber professional). Front Digit Health 2022;4:862221. [Crossref] [PubMed]
- Hirko KA, Kerver JM, Ford S, et al. Telehealth in response to the COVID-19 pandemic: Implications for rural health disparities. J Am Med Inform Assoc 2020;27:1816-8. [Crossref] [PubMed]
- Cheng J, Arora VM, Kappel N, et al. Assessing Disparities in Video-Telehealth Use and eHealth Literacy Among Hospitalized Patients: Cross-sectional Observational Study. JMIR Form Res 2023;7:e44501. [Crossref] [PubMed]
- Fix OK, Serper M. Telemedicine and Telehepatology During the COVID-19 Pandemic. Clin Liver Dis (Hoboken) 2020;15:187-90. [Crossref] [PubMed]
- Talal AH, Sofikitou EM, Jaanimägi U, et al. A framework for patient-centered telemedicine: Application and lessons learned from vulnerable populations. J Biomed Inform 2020;112:103622. [Crossref] [PubMed]
- Terry DL, Buntoro SP. Perceived Usefulness of Telehealth Among Rural Medical Providers: Barriers to Use and Associations with Provider Confidence. J Technol Behav Sci 2021;6:567-71. [Crossref] [PubMed]
- Gajarawala SN, Pelkowski JN. Telehealth Benefits and Barriers. J Nurse Pract 2021;17:218-21. [Crossref] [PubMed]
- Shaw J, Brewer LC, Veinot T. Recommendations for Health Equity and Virtual Care Arising From the COVID-19 Pandemic: Narrative Review. JMIR Form Res 2021;5:e23233. [Crossref] [PubMed]
- Nouri S, Khoong EC, Lyles CR, et al. Addressing Equity in Telemedicine for Chronic Disease Management During the Covid-19 Pandemic. NEJM Catalyst 2020;1.
- Adepoju OE, Chae M, Ojinnaka CO, et al. Utilization Gaps During the COVID-19 Pandemic: Racial and Ethnic Disparities in Telemedicine Uptake in Federally Qualified Health Center Clinics. J Gen Intern Med 2022;37:1191-7. [Crossref] [PubMed]
- Buchanan S, Peixoto C, Belanger C, et al. Investigating Patient Experience, Satisfaction, and Trust in an Integrated Virtual Care (IVC) Model: A Cross-Sectional Survey. Ann Fam Med 2023;21:338-40. [Crossref] [PubMed]
- Hayes CJ, Gannon MA, Woodward EN, et al. Implementation and Preliminary Effectiveness of a Multidisciplinary Telemedicine Pilot Initiative for Patients with Chronic Non-Cancer Pain in Rural and Underserved Areas at a Major Academic Medical Center. J Pain Res 2023;16:55-69. [Crossref] [PubMed]
- Mao A, Tam L, Xu A, et al. Barriers to Telemedicine Video Visits for Older Adults in Independent Living Facilities: Mixed Methods Cross-sectional Needs Assessment. JMIR Aging 2022;5:e34326. [Crossref] [PubMed]
- Kalicki AV, Moody KA, Franzosa E, et al. Barriers to telehealth access among homebound older adults. J Am Geriatr Soc 2021;69:2404-11. [Crossref] [PubMed]
- Utley LM, Manchala GS, Phillips MJ, et al. Bridging the Telemedicine Gap Among Seniors During the COVID-19 Pandemic. J Patient Exp 2021;8:23743735211014036. [Crossref] [PubMed]
- Chu JN, Kaplan C, Lee JS, et al. Increasing Telehealth Access to Care for Older Adults During the COVID-19 Pandemic at an Academic Medical Center: Video Visits for Elders Project (VVEP). Jt Comm J Qual Patient Saf 2022;48:173-9. [Crossref] [PubMed]
- Henson JB, Wegermann K, Patel YA, et al. Access to technology to support telehealth in areas without specialty care for liver disease. Hepatology 2023;77:176-85. [Crossref] [PubMed]
- Williams C, Shang D. Telehealth Usage Among Low-Income Racial and Ethnic Minority Populations During the COVID-19 Pandemic: Retrospective Observational Study. J Med Internet Res 2023;25:e43604. [Crossref] [PubMed]
- Brewster RCL, Zhang J, Stewart M, et al. A Prescription for Internet: Feasibility of a Tablet Loaner Program to Address Digital Health Inequities. Appl Clin Inform 2023;14:273-8. [Crossref] [PubMed]
- Qin J, Chan CW, Dong J, et al. Telemedicine is associated with reduced socioeconomic disparities in outpatient clinic no-show rates. J Telemed Telecare 2023; Epub ahead of print. [Crossref] [PubMed]
- Wegermann K, Wilder JM, Parish A, et al. Racial and Socioeconomic Disparities in Utilization of Telehealth in Patients with Liver Disease During COVID-19. Dig Dis Sci 2022;67:93-9. [Crossref] [PubMed]
- Chen A, Ayub MH, Mishuris RG, et al. Telehealth Policy, Practice, and Education: a Position Statement of the Society of General Internal Medicine. J Gen Intern Med 2023;38:2613-20. [Crossref] [PubMed]
- Mehrotra A, Nimgaonkar A, Richman B. Telemedicine and Medical Licensure - Potential Paths for Reform. N Engl J Med 2021;384:687-90. [Crossref] [PubMed]
- Barua I, Vinsard DG, Jodal HC, et al. Artificial intelligence for polyp detection during colonoscopy: a systematic review and meta-analysis. Endoscopy 2021;53:277-84. [Crossref] [PubMed]
- Hirten RP, Danieletto M, Scheel R, et al. Longitudinal Autonomic Nervous System Measures Correlate With Stress and Ulcerative Colitis Disease Activity and Predict Flare. Inflamm Bowel Dis 2021;27:1576-84. [Crossref] [PubMed]
- Pimentel M, Mathur R, Wang J, et al. A Smartphone Application Using Artificial Intelligence Is Superior To Subject Self-Reporting When Assessing Stool Form. Am J Gastroenterol 2022;117:1118-24. [Crossref] [PubMed]
Cite this article as: Aldzhyan V, Tamamian C, Tabibian JH. Leveraging telemedicine in gastroenterology and hepatology: a narrative review. mHealth 2023;9:36.