Designing and pilot-testing SmokefreeSGM: a text-based smoking cessation intervention for sexual and gender minority groups
Highlight box
Key findings
• The findings from our pilot test suggest that SmokefreeSGM is usable and acceptable among SGM smokers who want to quit smoking. Additionally, the findings from our pilot test show higher engagement among participants with the tailored bidirectional text messages (54%), specific to SmokefreeSGM, than the non-tailored bidirectional text messages (41.9%).
What is known and what is new?
• No text-based smoking cessation intervention has been specifically tailored to SGM smokers.
What is the implication, and what should change now?
• Findings from the pilot test will help us refine the SmokefreeSGM program and study procedures in preparation for a feasibility trial that will determine its viability and practicality.
Introduction
Background and rationale
Sexual and gender minority (SGM) groups—which include but are not limited to lesbian, gay, bisexual, transgender, and queer individuals—have a higher prevalence of cigarette smoking than heterosexual individuals. Cigarette smoking among lesbian, gay, and bisexual adults is approximately 1.3 times higher than among straight adults; while cigarette smoking among transgender, gender-expansive, and nonbinary adults is approximately 1.7 times higher than cisgender adults (1,2). Several factors account for the increased prevalence of cigarette smoking among this population, including minority-specific stressors (e.g., adoption of heterosexist attitudes, stigma, gender identity concealment, homophobia, discrimination) and targeted tobacco marketing (3,4). As a result, this population is at greater risk for developing tobacco-related health conditions including cancer, heart disease, and stroke, among others. However, there are few smoking cessation interventions that address the specific needs of SGM smokers, let alone tailored and personalized mobile health (mHealth) interventions that can be scalable at a relatively low cost. It has been suggested that SGM-tailored interventions could be more effective among this population because they can provide a validating environment that enhances responsiveness to cessation (5).
The rapid growth of mobile phone ownership, especially among marginalized populations, has expanded access to behavioral change interventions (6). SGM individuals encounter additional barriers to smoking cessation interventions due to factors such as low health insurance rates and inadequate cultural competency in the health care system (7). Therefore, a text-based program that allows for self-initiation and self-management could be an effective means of reducing tobacco-related health disparities among this population.
SmokefreeTXT is a text-based smoking cessation intervention developed by the National Cancer Institute (NCI) for the general population. The automated service provides evidence-based support, encouragement, and advice for quitting smoking over 8 weeks. It also offers on-demand support through the use of keywords (i.e., CRAVE, MOOD, SLIP) in which users can get additional messages outside of the main storyline when needed. SmokefreeTXT has been successfully adapted by NCI for pregnant women, teens, and military veterans (8-10). As a result, it provides a solid foundation upon which an SGM-tailored version of the program could be developed. Therefore, the objective of this phase of our study was twofold: (I) to develop SmokefreeSGM, a tailored text-based smoking cessation program for SGM smokers, and (II) to pilot test the design of SmokefreeSGM among 18 SGM smokers through a mixed-methods approach that will inform the refinement of the text-based smoking cessation program prior to launching a feasibility trial with a larger sample. We present this article in accordance with the COREQ reporting checklist (available at https://mhealth.amegroups.com/article/view/10.21037/mhealth-23-4/rc).
Methods
Developing the SmokefreeSGM library
A community-based participatory research (CBPR) approach was used for developing the SmokefreeSGM text library. CBPR integrates the knowledge of a community to address health disparities and improve health outcomes (11). Thus, the original SmokefreeTXT library of text messages, developed for the general population, was tailored to SGM smokers with input and feedback from members of an Advisory Committee composed of SGM former and current smokers, smoking cessation specialists, as well as scientists and community leaders, many of who self-identify as SGM individuals, with whom our research team has collaborated with in previous research and advocacy efforts around SGM health disparities research. While the SmokefreeSGM library includes some of the same text messages as SmokefreeTXT, others were tailored to resonate with SGM groups (Table 1). Furthermore, text messages are sent by “Alex”, a fictitious SGM peer ex-smoker quit coach with a gender-neutral name. The original keywords from the SmokefreeTXT library were also kept for on-demand support, but a new keyword, STRESS, was added to prompt an additional set of text messages that address unique psychosocial stressors for SGM smokers. STRESS, CRAVE, and MOOD can be used by the participant if they need additional encouragement to remain smoke free. The SmokefreeSGM library has 98 unidirectional and 37 bidirectional text messages. Unidirectional or one-way text messages in the SmokefreeSGM program refer to those text messages sent to the user, which do not require or allow a response. We utilized bidirectional or two-way text messages as a means of increasing user engagement in the program by tailoring the responses to the user and how they are currently feeling. Participants were asked to respond to a question from “Alex” from choices outlined in the message (e.g., reply with: HARD, SO-SO, or EASY). Based on the answer received, “Alex” would respond with a personalized message (Table 1).
Table 1
| Type of tailoring | Sample text messages |
|---|---|
| Tailoring SmokefreeTXT to SGM smokers | QuitNowTXT: Stress and anger are smoking triggers. If you’re feeling stressed or upset to get extra support; call or text a friend or family member to lean on |
| Alex: Stress and discrimination are smoking triggers for LGBTQ+ people. If you’re stressed out or upset, call or text a friend or chosen family to lean on | |
| QuitNowTXT: We know quitting is hard and sometimes it takes a few tries. Do you want to continue or start over and set a new quit date? Reply: STAY or NEW | |
| Alex: Quitting is a process, like coming out, sometimes it takes a few tries. Do you want to continue or start over and set a new quit date? Reply: STAY or NEW | |
| QuitNowTXT: Smoking is like a bad romance, you have to know when to walk away! Don’t sit around missing your old cigs. Curl up with a movie or a book instead | |
| Alex: Smoking is like a bad romance, you have to walk away! Don’t sit around missing your old cigs. Curl up with your favorite queer movie or a book instead | |
| SmokefreeSGM-bidirectional text messages | Alex: Sexual orientation concealment refers to hiding one’s true sexual identity. Please rank how stressful this is on a scale from 1 (not) to 5 (very) |
| Alex: Coming out to friends or family can be a journey for many LGBTQ+ people. Please rank how stressful this is on a scale from 1 (not) to 5 (very) | |
| Alex: Internalized homophobia are beliefs about homophobic lies, stereotypes and myths. Please rank how stressful this is on a scale from 1 (not) to 5 (very) | |
| STRESS keyword | Alex: LGBTQ+ folks report high rates of stress. How are you? Are you feeling stressed today? Reply STRESSED or CALM |
| • STRESSED—Sorry to hear! Focus on your strengths; resilience takes practice like reminding yourself about your strengths. List 3 things you’ve done today | |
| • CALM—That’s awesome, keep that sunny disposition in your back pocket for a rainy day | |
| Alex: Negative self-talk can be a barrier to feeling good. We need to love ourselves! Are you feeling down on yourself today? Reply STRESSED or CALM | |
| • STRESSED—It can be a journey to self-acceptance. Curb negativity and work towards positivity, say: today, I love and accept myself. Your effort shows your heart | |
| • CALM—Glad to hear it! You’re your best bet at success! A can-do mentality will keep your heart open and your body healthy |
SGM, sexual and gender minorities; LGBTQ, lesbian, gay, bisexual, transgender.
The readability of each SmokefreeSGM text message was calculated using the Flesch-Kincaid Grade Level and Dale-Chall score. The Flesch-Kincaid Grade Level assesses the approximate U.S. reading grade level of text based on sentence length (avg. number of words in a sentence) and word length (avg. number of syllables in a word). The formula calculates a score that corresponds with a U.S. grade level (12). The Dale-Chall score assesses the readability of text based on a list of 3,000 words commonly understood by 4th grade students. The formula calculates a number based on the percentage of words in the text that are not found on the list, which is adjusted to correspond with a U.S. grade level (13). These measures helped us determine what if any changes needed to be made to ensure users’ comprehension of the text messages. When calculating the Flesch-Kincaid scores, the average score for the entire library was 4.2 (±2.32), indicating that it could be easily understood by the average 4th grade student. The average Dale-Chall score for the entire library was 6.8 (±1.87), indicating that it could be easily understood by the average 7th or 8th grade student (13). The discrepancy in the grade levels is due to the variables calculated in the formulas (word and sentence length vs. word choice). When developing the text library, the research team attempted to get the lowest Flesch-Kincaid and Dale-Chall score for each text message without undermining its content.
Building the SmokefreeSGM text-based platform
The SmokefreeSGM text library was input into an automated text messaging software designed for health research. Following participants’ enrollment into the study, their cell phone number was entered into the software and the storyline was initiated. Both unidirectional and bidirectional text messages were sent to users daily for a 6-week period: 2 weeks prior to their quit date, on their quit date, and 4 weeks after their quit date. All participants received the same number of text messages in the same sequence. However, the content of some messages varied depending on their responses to the bidirectional text messages (see Table 1). Additionally, a bidirectional message assessing smoking status was sent at 1-, 3-, and 6-months after participants’ quit date (e.g., “Are you smokefree or back to smoking? Reply with FREE or BACK”). However, for the purpose of this pilot test, only responses received during the 1-month assessment were used to assess smoking abstinence (exploratory outcome).
Pilot testing the SmokefreeSGM intervention
The objective of this phase of our study is to test the SmokefreeSGM text messaging platform to assess its usability and acceptability as well as evaluate our study procedures before launching our feasibility trial among a larger sample.
Recruitment procedures
A printed version of the recruitment flyer was distributed at small businesses in Houston, Texas (e.g., coffee shops, restaurants, boutiques, bars, etc.) as well as at the 2022 Houston Pride Festival. An electronic version of the flyer was also sent to local community organizations and healthcare facilities working for or providing services to SGM groups. The flyer included information for contacting the study team via phone call, email, and/or by completing an electronic “Contact Us” form in REDCap, an application designed to support data collection for research studies. Our research team also utilized ResearchMatch, a program funded by the National Institutes of Health (NIH), to connect us with individuals who may be interested in participating in our pilot test.
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Informed consent was obtained from all study participants. The study was approved by the Institutional Review Board (IRB) of The University of Texas Health Science Center at Houston (No. HSC-SPH-20-0318).
Study population
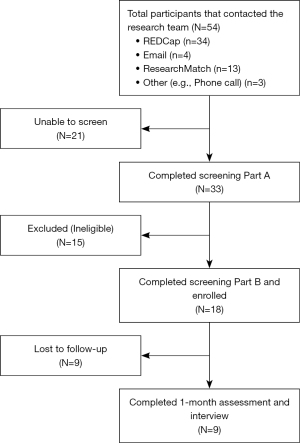
Fifty-four individuals contacted our research team, 18 SGM smokers were enrolled, and 9 completed the pilot test (see Figure 1). The first participant was enrolled in January 2022 and the last participant was enrolled in September 2022.
The inclusion criteria were as follows: (I) self-identify as an SGM individual; (II) age ≥18 years; (III) currently (in the past 30 days) smoke every day and smoke five or more cigarettes per day; (IV) are interested in quitting smoking in the next 15 days; (V) have a cellphone number with an unlimited short messaging service (SMS) plan; (VI) have a US mailing and email addresses; and (VI) have positive cotinine saliva test results for biologically confirming current smoking status.
SGM smokers who did not understand English were excluded as the SmokefreeSGM program is only available in English at this time. Individuals who were found to have a prepaid cell phone (pay-as-you-go plan), a cellphone number that does not work or a cellphone number that is registered to someone else were excluded. Potential participants with absolute contraindications for the nicotine patch (e.g., severe eczema or serious skin conditions, allergy to nicotine patch, pregnancy, breastfeeding, heart attack in the past 2 months, ongoing angina, peptic ulcer disease, arrhythmia, or uncontrolled blood pressure) were ineligible for the study. Individuals reporting a stroke in the past 6 months, receiving insulin therapy, or recently diagnosed with liver, kidney, or heart disease were required to receive approval from their primary care provider and/or other treating physician for using nicotine patches. If the written request from the healthcare providers was denied or not returned within 2 weeks, those potential participants were excluded from the study.
Two-step screening
We implemented a two-step screening procedure for individuals interested in participating in our pilot test. During Screening Part A (conducted over the phone), individuals were asked demographic, medical history, and tobacco use questions. Those deemed eligible to participate were consented electronically and invited to complete Screening Part B via video conference (i.e., WebEx) 7 days later, in which their self-reported smoking status would be verified by a saliva cotinine test (i.e., NICDetect, Alere) that was mailed to their home address. During Screening Part B, the saliva cotinine test was conducted by the potential study participant following detailed instructions provided by a research team member, who closely monitored the procedure. The saliva cotinine test required the individual to swab the inside of their mouth and tongue for 3 min before placing the collection sponge into the screening device. While waiting for the results, the research team member played two videos: the first one with information about the study and the second one with instructions for using nicotine patches. The results of the saliva cotinine test were available when a colored band appeared on the screening device approximately 10 min later, which was recorded by the research team member. Those with a positive result were eligible for the study and to continue with the baseline assessment, after which their phone number was entered into the storyline of the text messaging program. Those potential participants with a negative result were ineligible to participate in the study.
Baseline assessment
The baseline assessment, which was conducted during the same meeting as the second screening, included items to evaluate the demographics (e.g., age, sex assigned at birth, sexual orientation, gender identification, race/ethnicity, education, etc.) and smoking characteristics of participants (e.g., cigarettes smoked per day, past quit attempts, nicotine dependence, use of other tobacco products, etc.). SGM individuals who smoked 10 or fewer cigarettes per day were categorized as “Light Smokers”, while those who smoked more than 10 cigarettes per day were categorized as “Heavy Smokers”. This categorization was based on the NicoDerm CQ patch program in which heavy smokers have a 10-week treatment course starting with 21 mg patches and light smokers have an 8-week course starting with 14 mg patches (14). Following the baseline assessment, study participants were mailed the first 6-week supply of nicotine patches. Light smokers received 3 boxes of 14 mg patches and heavy smokers received 3 boxes of 21 mg patches. In addition to nicotine patches, study participants were also emailed a $15 electronic gift card as compensation.
1-month assessment
The 1-month assessment was also conducted remotely via video conference 6 weeks after enrollment (baseline assessment) and 4 weeks after the participants’ quit date. Participants’ engagement rates were ascertained at this time by dividing the total number of participant responses to the bidirectional messages (numerator) with the total number of bidirectional messages sent by the text-based platform (denominator). Participants received 28–31 bidirectional text messages depending on their responses to questions about their smoke free status. Participants who had rates ≤33.3% were classified as having low engagement, 33.3–66.6% moderate engagement and ≥66.7% high engagement. This information was used to ascertain the overall engagement rate for the program. At the 1-month assessment, Sexual Orientation and Gender Identity (SOGI) were reassessed to account for changes and tobacco use questions were posed again to determine participants’ current smoking status.
Additionally, the 10-item System Usability Scale (SUS) was measured to assess the usability of the SmokefreeSGM text messaging program [strongly disagree (1) to strongly agree (5)] and thus determine where improvements were needed. The questions posed to participants can be found in Table 2.
Table 2
| Item | Question | Generated response |
|---|---|---|
| 1 | Do you think that you would like to use the SmokefreeSGM texts frequently? | Positive |
| 2 | Did you find the text messages in the SmokefreeSGM program to be unnecessarily complex? | Negative |
| 3 | Did you find the SmokefreeSGM program to be easy to use? | Positive |
| 4 | Do you think that you would need the support of a technical person to be able to use the SmokefreeSGM program? | Negative |
| 5 | Did you find that the bidirectional messages in the SmokefreeSGM program were well integrated? | Positive |
| 6 | Did you think there was too much inconsistency in the SmokefreeSGM program? | Negative |
| 7 | Would you imagine that most people would learn how to use the SmokefreeSGM program quickly? | Positive |
| 8 | Did you find the SmokefreeSGM program cumbersome to use? | Negative |
| 9 | Did you feel confident using the SmokefreeSGM program? | Positive |
| 10 | Did you need to learn a lot of things before you could get started with the SmokefreeSGM program? | Negative |
We added the scores for all odd-numbered questions, which generate a positive response, and subtracted 5 from the total to get X. We then added up the scores for all even-numbered questions, which generate a negative response, and subtracted the total from 25 to get Y. The SUS score was ascertained by adding up the total score of the new values (X and Y) and multiplying the result by 2.5. A score above 75 indicates that the program is perceived as acceptable (15).
The 1-month assessment also included a qualitative semi-structured interview for assessing the usability and acceptability of SmokefreeSGM. Interview questions were based on the 10-items SUS scale where participants were asked “Why did you assign this many points to this question?” to obtain the corresponding qualitative data. Each semi-structured interview lasted for an average of 30 min. During the semi-structured interviews, there was nobody else present aside from the participants and researchers. The participants had no prior relationship with the interviewers and no negative interviewer characteristics, such as bias, were reported.
Study participants were also asked about how often they used nicotine patches over the past week. Following this session, they were mailed the second 2- or 4-week supply of nicotine patches. Light smokers received 1 box of 7 mg patches, and heavy smokers received 1 box of 14 mg patches and 1 box of 7 mg patches. While participants completed their involvement in the study following this assessment and interview, it was important that we provided the full course of nicotine replacement therapy (NRT) to assist them in their efforts to quit smoking. However, the second shipment of nicotine patches were not sent to those participants that did not complete the 1-month assessment nor participate in the interview. Additionally, individuals that participated in this session were emailed a $25 electronic gift card as compensation.
Statistical analysis
STATA/SE 17.0 software was used for quantitative analysis. The socio-demographic characteristics (e.g., age, gender identity, sexual orientation, race, ethnicity) of the 18 SGM smokers were assessed using descriptive statistics. Additionally, the tobacco use data was subjected to univariate analysis. Participants were categorized as having low, moderate, or high nicotine dependence based on their Fagerstrom Test for Nicotine Dependence (FTND) scores: less than 4, between 4 and 6, and greater than 6, respectively (16). The recruitment rate was calculated by dividing the number of participants enrolled into the study by the number of participants who contacted the research team. The retention rate was calculated by dividing the number of participants who completed the 1-month assessment with the number of participants enrolled into the study. In addition to computing participants’ engagement rates (proportion of bidirectional text messages responded to), the rate of response for each bidirectional text message was computed by dividing the number of participants that responded to a particular bidirectional text message (numerator) with the total number of participants (denominator). We subsequently calculated the average response rate for the tailored bidirectional text messages that address unique psychosocial stressors for SGM smokers and the non-tailored bidirectional text messages. Furthermore, engagement rates were calculated for each of the keyword storylines (i.e., STRESS, CRAVE, MOOD) to determine what percent of the study population utilized on-demand support. As for the usability of the program, participants’ scores were pooled to calculate the average SUS score for the study sample.
Audio recordings from the individual interviews were transcribed and analyzed by organizing and labeling relevant data into codes, allowing for the exploration of a priori concepts and for new themes to emerge. The thematic analysis was manually performed by two independent coders trained in qualitative methods. Twenty-one codes emerged from this process and were grouped into four major themes.
Results
The recruitment rate for the study was 33.3% while the retention rate was 50%. Nine participants completed the 1-month follow-up session, which involves a quantitative assessment and a qualitative individual interview.
Sociodemographic information
The study sample’s average age was 39 years (±12.16). Seven participants were male, seven were female, and four were nonbinary, genderfluid, or genderqueer. Five participants identified as gay men or men who have sex with men (MSM), two as lesbian, gay women, or women who have sex with women (WSW), three as bisexual males, five as bisexual females, and three as other sexual orientations (i.e., queer). Two participants were transgender individuals, while the other sixteen were cisgender individuals. We did not observe any SOGI changes among participants at the 1-month assessment. In our study sample, half of the study participants (50.0%) were non-Hispanic white. Most of the participants (72.2%) worked full-time. About three-quarters (72.2%) had some college education or less. Except for one study participant, all were either single, separated, widowed, or divorced (94.4%). The majority of the study participants (83.3%) did not have children living in their households. More information can be found in Table 3.
Table 3
| Characteristics | Value (n=18) |
|---|---|
| Age (years) | 39 (±12.16) |
| Gender identity | |
| Male | 7 (38.9) |
| Female | 7 (38.9) |
| Nonbinary, genderfluid, or genderqueer† | 4 (22.2) |
| Sexual orientation | |
| Lesbian/gay woman/WSW | 2 (11.1) |
| Gay man/MSM | 5 (27.8) |
| Bisexual femalea | 5 (27.8) |
| Bisexual Maleb | 3 (16.7) |
| Other‡ | 3 (16.7) |
| Race and ethnicity | |
| Hispanic | 4 (22.2) |
| Non-Hispanic White | 9 (50.0) |
| Non-Hispanic Black | 3 (16.7) |
| Non-Hispanic other§ | 2 (11.1) |
| Work status | |
| Not working | 1 (5.6) |
| Working full time | 13 (72.2) |
| Working part-time | 4 (22.2) |
| Education | |
| Some college or less | 13 (72.2) |
| College and higher | 5 (27.8) |
| Marital status | |
| Single/separated/divorced/widowed | 17 (94.4) |
| Married/living with significant other | 1 (5.6) |
| Children in household | |
| Yes | 3 (16.7) |
| No | 15 (83.3) |
Data are shown as average (± standard deviation) or n (%). †, included one male-to-female transgender and one female-to-male transgender; ‡, included Pansexual and Queer; §, includes Asian, American Indian, Native Hawaiian; a, included one transgender; b, included one transgender. WSW, women who have sex with women; MSM, men who have sex with men.
Tobacco-related characteristics
At baseline, the SGM participants smoked an average of 15 cigarettes per day. The average age at which they first smoked was 14.8 (±2.96) years. Only two participants (11.1%) lived with other smokers. Eight participants (44.4%) had tried to quit smoking more than five times, nine participants (50.0%) had tried between one and five times, and only one (5.6%) participant had never attempted to quit smoking. Based on FTND scores obtained at baseline, 27.8% of participants had a high dependence on nicotine, 38.9% a moderate dependence, and 33.3% a low dependence at the start of the study. About two-fifths (38.9%) of participants were heavy smokers (>10 cigarettes/day), while the remaining 61.1% were light smokers (≤10 cigarettes/day). At the 1-month assessment, 85.7% of participants had used nicotine patches within the past week. While not a primary outcome of this pilot test, based on responses to the smoke free status text message sent to participants at 1-month post quit date (“Are you smokefree or back to smoking? Reply with FREE or BACK”), 9 of the 12 participants (75%) that responded reported that they were currently smoke free.
Quantitative assessment (engagement and usability)
The average engagement rate was 63.8%, indicating that participants responded to about two-thirds of all bidirectional text messages sent to them. About 55.6% of participants had high engagement, 16.7% moderate engagement, and 27.7% low engagement. The average response to the tailored bidirectional text messages that address unique psychosocial stressors for SGM smokers was 54.0% while the rate for the non-tailored bidirectional text messages from SmokefreeTXT was 41.9% (Table 3). Three participants (16.7%) used the new keyword, STRESS, while no participants used the other keywords (CRAVE and MOOD). More information can be found in Table 4.
Table 4
| Keywords | Text messages | Rate |
|---|---|---|
| Stress | Coming out to friends or family can be a journey for many LGBTQ+ people. Please rank how stressful this is on a scale from 1 (not) to 5 (very) | 81.25% |
| Sexual orientation concealment refers to hiding one’s true sexual identity. Please rank how stressful this is on a scale from 1 (not) to 5 (very)—10 days prior to quit date | 62.50% | |
| Discrimination refers to unfairly treating a person based on their sexual orientation. Please rank how stressful this is on a scale from 1 (not) to 5 (very) | 50% | |
| Sexual orientation concealment refers to hiding one’s true sexual identity. Please rank how stressful this is on a scale from 1 (not) to 5 (very)—4 days prior to quit date | 68.75% | |
| Internalized homophobia are beliefs about homophobic lies, stereotypes and myths. Please rank how stressful this is on a scale from 1 (not) to 5 (very) | 37.50% | |
| Alex: Use the keyword STRESS if you are feeling stressed out and need support. Are you feeling stressed today? Reply STRESSED or CALM | 50% | |
| Alex: LGBTQ+ folks report high rates of stress. How are you? Are you feeling stressed today? Reply STRESSED or CALM | 56.25% | |
| Alex: Are you tempted to light up that cigarette to cope with stress from stigma, prejudice, and/or discrimination? Reply STRESSED or CALM | 50% | |
| Alex: This year has been challenging for everyone. Do feelings of isolation/loneliness contribute to your stress levels? Reply STRESSED or CALM | 43.75% | |
| Alex: While your sexual identity is important for who you are, you are so much more than that! Have you felt less like yourself lately? Reply: STRESSED or CALM | 50% | |
| Alex: Due to stigma, LGBTQ+ folks often wrestle with fear of rejection in their personal lives and at work. Do you ever feel that way? Reply STRESSED or CALM | 43.75% | |
| Average | 54.0% | |
| Crave | Alex: Cravings are real. They won’t go away immediately, but feeding them only makes them stronger. What is your craving level? Reply: HI, MED, or LOW | 37.50% |
| Alex: Wait 5 minutes for cravings to pass. Keep your mouth busy. What is your craving level? Reply: HI, MED, or LOW | 43.75% | |
| Alex: Cravings will get weaker and less frequent with every day that you don’t smoke. What is your craving level? Reply: HI, MED, or LOW | 37.50% | |
| Alex: To deal with cravings: breathe in, hold for 5 seconds, breathe out, and repeat. What is your current craving level? Reply: HI, MED, or LOW | 37.50% | |
| Alex: Having just one puff of a cigarettes will only feed your cravings and make them stronger. What is your craving level today? Reply: HI, MED, or LOW | 37.50% | |
| Alex: Day 25! Congratulations! By this time, most people cravings start to fade. What is your craving level? Reply: HI, MED, or LOW | 25% | |
| Mood | Alex: If you’re feeling cranky it could be because you’re quitting smoking. This is only temporary, so stay strong! Reply with your mood: GOOD, OK, or BAD | 37.50% |
| Alex: It has been 9 days since you quit smoking. Congratulations! How are you feeling today? Text back: GOOD, OK, or BAD | 37.50% | |
| Alex: How are you feeling today? Reply with: GOOD, OK, or BAD—Day 2 post quit date | 68.75% | |
| Alex: How are you feeling today? Reply with: GOOD, OK, or BAD—Day 10 post quit date | 56.25% | |
| Average | 41.9% |
The SUS score for SmokefreeSGM was 81.7 (±15.46), indicating high perceived usability among participants.
Qualitative assessment (usability and acceptability)
After analyzing the transcripts of the 9 participants who participated in the qualitative interviews, four major themes emerged from the coding process: appreciation, usability, content, and drawbacks. These themes are important for determining what improvements to SmokefreeSGM are needed prior to launching our feasibility trial with a larger sample.
Theme 1: appreciation of the text-based program
This theme included five codes: encouragement, appreciation, timing of texts (positive), managing cravings, and reminders. The theme encompasses positive feedback about the program’s features and content.
“…I thought about each message [from SmokefreeSGM]. It actually gave me the encouragement to not want to smoke.”—Gay male, 34, Black, heavy smoker.
“…and in the evenings, they [SmokefreeSGM] would send that text and it’s like … okay, I’ll remember why I’m doing this. I think they [the text messages] were very helpful.”—Bisexual female, 29, White, light smoker.
“I haven’t completely stopped, but I have slowed down. I do see some progress in not going for cigarettes.” —Gay male, 34, Black, heavy smoker.
Theme 2: usability of the text-based program
This theme describes participants’ perceived usability of the program and includes five codes: simple instructions, clear instructions, teachable, convenient, and daily texts.
“I do not feel that [the text messages] were complex … I would say that the general public would be able to understand the texts.” —Bisexual female, 47, White, heavy smoker.
“It is simple, it’s straightforward, it’s not rocket science, … It’s easy and it’s not overwhelming at all.” —Lesbian female, 32, Hispanic, light smoker.
“I check the text message whenever it’s convenient for me.” —Gay male, 58, Hispanic, heavy smoker.
Theme 3: SmokefreeSGM texts’ content
Five codes made up this theme: SGM content, knowledge content (related to the health implications of tobacco use), suggested improvements, comments about Alex, and text versus app-based programs. This theme details the program’s subject matter and associated opinions.
“I think it was easy to use, not cumbersome because it wasn’t an app.” —Bisexual female, 29, White, light smoker.
“If it [SmokefreeSGM] could have more of perception-raising or insight-raising, I think that could add to … benefit to the user.” —Bisexual female, 47, White, heavy smoker.
“Having an automated text is just like talking to a real person … and that’s really helpful for somebody like me.” —Lesbian female, 32, Hispanic, light smoker.
“[The program] was consistently asking how I was feeling, or it would give me inspiration, specifically geared toward smoking. You know, information about how LGBT [individuals are] affected by [smoking] more and stuff like that, so I think it … kept [me] on course.” —Gay male, 35, White, heavy smoker.
“I also enjoyed the couple of facts, you know? The stuff like ‘your night vision gets better’ and then the unfortunate facts about how [SGM] have it worse off, pretty much, in the smoking world.” —Bisexual female, 29, White, light smoker.
Theme 4: drawbacks
Six codes were included in this theme: difficult to use, timing of texts (negative feedback), unclear instructions, inadequate bidirectional conversations, overwhelming content, and discouraging content. This theme includes issues participants experienced with the program and features they disliked.
“I did get pushed at for quitting … it was at times overwhelming,” —Bisexual female, 38, White, light smoker.
“There wasn’t much informing about [the use of keywords: MOOD, STRESS, CRAVE]. That’s why I rarely used that feature, like they didn’t explain that you could and I don’t know still.” —Gay male, 35, White, heavy smoker.
“[The texts] were like don’t worry about adding extra pounds, and I kind of was worrying about adding extra pounds.” —Bisexual female, 29, White, light smoker.
Discussion
Principal findings
The pilot test of SmokefreeSGM found that the text messaging program had a high perceived usability and acceptability, as well as sufficient engagement. Furthermore, our quantitative and qualitative findings provided insights into how both the text messaging program and study procedures could be improved upon prior to launching a feasibility trial among a larger sample. The SUS score of the SmokefreeSGM program was 81.7 (±15.46), higher than the 75-percentile benchmark for high perceived usability (16). Similar to previous qualitative studies on text-based interventions, participants also reported that SmokefreeSGM was easy to use (17-19). This suggests that our program provides the ease of use that SGM individuals require to navigate the smoking cessation process.
SmokefreeSGM, like SmokefreeTXT, provides bidirectional text messages for participants to enhance their interaction with the program. However, the findings from our pilot test show higher engagement among participants with the tailored bidirectional text messages (54%), specific to SmokefreeSGM, than the non-tailored bidirectional text messages (41.9%), adapted from the original SmokefreeTXT program. This indicates that study participants are more engaged with SGM-specific content. This could positively impact the efficacy of the SmokefreeSGM program as it relates to smoking abstinence. This will be further explored during our feasibility trial in which engagement rates with SmokefreeSGM (intervention arm) will be directly compared to SmokefreeTXT (control arm) and smoking abstinence data will be collected at 1-, 3-, and 6-months follow-up among all study participants. Furthermore, a study comparing engagement of Black and White SmokefreeTXT users reported engagement rates ranging between 6% to 17% for Blacks and 8% to 25% for whites (20,21). Accordingly, when compared to SmokefreeTXT, our program reported higher engagement.
Only three participants (16.7%) in our study sample used the keywords (i.e., STRESS, CRAVE, MOOD) for on-demand support, which made it clear that our research team needs to emphasize this aspect of the program. As a result, we have reviewed our instructional materials and made edits to the educational videos shown during the Screening Part B. We also created laminated cards explaining how to utilize on-demand support and the purpose of each keyword, which will be sent to enrolled participants along with their shipments of nicotine patches. Neither of these changes were implemented during the pilot test. However, as mentioned above, they will be implemented for the feasibility trial.
As it relates to the content of the SmokefreeSGM tailored text messages, a majority of participants found it acceptable, and no suggestions were made concerning its cultural competency. Therefore, few if any revisions will be required for subsequent iterations of the program. However, a number of suggestions were made about the timing of text messages received throughout the day (7 am, 12 pm, 7 pm). Some participants claimed that the timing was ideal, while others suggested that having the ability to customize when they received text messages would be beneficial, which is similar to findings from the MiQuit text-based smoking cessation program for pregnant smokers (22). While it is unlikely that we will be able to implement this change for our feasibility trial, it will be important for future iterations of the program and related research efforts.
Another change we intend to implement for the feasibility trial is expanding the eligibility criteria to allow dual users (individuals who smoke cigarettes and use electronic cigarettes) to participate in the study. It is estimated that approximately 40% of electronic cigarette users are also cigarette smokers (23). During our initial screenings, many individuals were deemed ineligible to participate for this reason. Implementing this change will allow us to expand our study to a larger population, while at the same time assess the impact of SmokefreeSGM on dual users.
Limitations and recommendations for optimization
This pilot study had a few limitations, which have informed the optimization of the SmokefreeSGM program. The pilot test of SmokefreeSGM was originally designed as an in-person study in which screenings, assessments, and interviews were conducted at The University of Texas Health Science Center at Houston School of Public Health. However, the coronavirus disease 2019 (COVID-19) pandemic required us to move our data collection efforts to a virtual environment. For the remote operation of this study, we had to mail saliva test kits and nicotine patches to participants’ home addresses and provide compensation with electronic gift cards sent via email. By revising our program to be sustainable during the pandemic, participants could schedule sessions in consideration of their availability and complete them in the comfort of their own homes. While these changes delayed our proposed timeline, it also provided us with an opportunity to expand recruitment from the Greater Houston Metropolitan Area to individuals across Texas, thus enhancing the diversity of our sample population and preparing us for the eventual expansion of this study nationwide. Therefore, despite the lifting of COVID-19 restrictions, our feasibility trial will remain remote.
We recorded a 50% loss to follow-up which was lower than what was reported for iQuit in Practice, a text-based facilitation of smoking cessation in primary care, after 4 weeks (69.9%) (24). While our retention rate may have been a result of our small sample size, it is possible that some participants were lost to follow-up because they did not want to report that they had been unable to quit smoking. Additionally, despite providing nicotine patches and a $25 electronic gift card for completion of the 1-month assessment, compensation may have been insufficient for some participants, which in itself is an important finding for the subsequent feasibility trial.
As for our study population, we recognize that excluding non-English speaking individuals, specifically Spanish speakers, from the SmokefreeSGM program limits the reach of our intervention, especially in Texas where the Latino community makes up 40.2% of the state’s population (25). Unfortunately, it was not feasible within the scope of this study. Subsequent research efforts and the expansion of this program nationwide will allow us to develop and deliver this program in both English and Spanish.
Conclusions
SmokefreeSGM has been tailored to address the unique needs and experiences of SGM smokers. The results from our pilot test are encouraging in terms of the program’s usability and acceptability but have also informed the refinement of our intervention. Our future research efforts, which include performing a feasibility trial to determine the viability and practicality of the program, will help address the high prevalence rates of cigarette and tobacco use among the SGM population. In doing so, we will also contribute to the body of evidence for mHealth behavioral change interventions.
Acknowledgments
We would like to acknowledge NCI’s SmokefreeTXT program as it served as the foundation for SmokefreeSGM and extend our thanks to the former students of the Health Equity Research Group, James Che, Andrea Melinger, Carolyn Crisp, Modupe Onigbogi, Mira Dani, Sindujha Mahalingam, and Niles Zoschke, for their invaluable support during the development and launching of our study.
We also want to acknowledge the contribution of the SGM current and former smokers, tobacco specialists, and scientists and clinicians engaged in SGM research that made up our Community Advisory Board. Their expertise was instrumental in the development of the SmokefreeSGM text messaging program. Finally, we would like to thank the study participants who provided their valuable feedback for improving the program.
Funding: This work was supported by a grant (No. 1K22CA237639) awarded by the National Institutes of Health (NIH) and National Cancer Institute (NCI) to Principal Investigator, Irene Tamí-Maury, DMD, DrPH, MSc of The University of Texas Health Science Center at Houston.
Footnote
Reporting Checklist: The authors have completed the COREQ reporting checklist. Available at https://mhealth.amegroups.com/article/view/10.21037/mhealth-23-4/rc
Data Sharing Statement: Available at https://mhealth.amegroups.com/article/view/10.21037/mhealth-23-4/dss
Peer Review File: Available at https://mhealth.amegroups.com/article/view/10.21037/mhealth-23-4/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://mhealth.amegroups.com/article/view/10.21037/mhealth-23-4/coif). ITM serves as an unpaid editorial board member of mHealth from December 2022 to November 2024. ITM reports a grant support from The National Institutes of Health (NIH) and National Cancer Institute (NCI) - 1K22CA 237639. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Informed consent was obtained from all study participants. The study was approved by the Institutional Review Board (IRB) of The University of Texas Health Science Center at Houston (No. HSC-SPH-20-0318).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Tan AS, Gazarian PK, Darwish S, et al. Smoking Protective and Risk Factors Among Transgender and Gender-Expansive Individuals (Project SPRING): Qualitative Study Using Digital Photovoice. JMIR Public Health Surveill 2021;7:e27417. [Crossref] [PubMed]
- Current Cigarette Smoking Among Adults — United States, 2005–2014. [cited 2022 Oct 6]. Available online: https://www.cdc.gov/mmwr/preview/mmwrhtml/mm6444a2.htm
- Meyer IH. Prejudice, social stress, and mental health in lesbian, gay, and bisexual populations: conceptual issues and research evidence. Psychol Bull 2003;129:674-97. [Crossref] [PubMed]
- Washington HA. Burning Love: big tobacco takes aim at LGBT youths. Am J Public Health 2002;92:1086-95. [Crossref] [PubMed]
- Berger I, Mooney-Somers J. Smoking Cessation Programs for Lesbian, Gay, Bisexual, Transgender, and Intersex People: A Content-Based Systematic Review. Nicotine Tob Res 2017;19:1408-17. [PubMed]
- Labrique AB, Vasudevan L, Kochi E, et al. mHealth innovations as health system strengthening tools: 12 common applications and a visual framework. Glob Health Sci Pract 2013;1:160-71. [Crossref] [PubMed]
- Pratt-Chapman ML, Eckstrand K, Robinson A, et al. Developing Standards for Cultural Competency Training for Health Care Providers to Care for Lesbian, Gay, Bisexual, Transgender, Queer, Intersex, and Asexual Persons: Consensus Recommendations from a National Panel. LGBT Health 2022;9:340-7. [Crossref] [PubMed]
- Christofferson DE, Hertzberg JS, Beckham JC, et al. Engagement and abstinence among users of a smoking cessation text message program for veterans. Addict Behav 2016;62:47-53. [Crossref] [PubMed]
- Abroms LC, Chiang S, Macherelli L, et al. Assessing the National Cancer Institute's SmokefreeMOM Text-Messaging Program for Pregnant Smokers: Pilot Randomized Trial. J Med Internet Res 2017;19:e333. [Crossref] [PubMed]
- Squiers L, Brown D, Parvanta S, et al. The SmokefreeTXT (SFTXT) Study: Web and Mobile Data Collection to Evaluate Smoking Cessation for Young Adults. JMIR Res Protoc 2016;5:e134. [Crossref] [PubMed]
- Community-Based Participatory Research Program (CBPR). [cited 2022 Oct 6]. Available online: https://www.nimhd.nih.gov/programs/extramural/community-based-participatory.html
- Kincaid P, Fishburne R, Rogers R, et al. Derivation of New Readability Formulas (Automated Readability Index, Fog Count and Flesch Reading Ease Formula) For Navy Enlisted Personnel. Research Branch Report. Insti-tute for Simulation and Training; 1975. Available online: https://stars.library.ucf.edu/istlibrary/56/
- Chall, D. Readability Revisited: The New Dale-Chall Readability Formula. Brookline. 1995. Available online: https://www.semanticscholar.org/paper/CHALL%2C-J.-S.-and-DALE%2C-E.-(1995)-Readability-%3A-The-%E6%B8%85%E5%B7%9D/841eac7dfa47e1fc7bda477ba88dfc1dd3f8a59d
- Nicotine (Transdermal Route) Proper Use - Mayo Clinic. [cited 2023 Feb 9]. Available online: https://www.mayoclinic.org/drugs-supplements/nicotine-transdermal-route/proper-use/drg-20068808
- Hyzy M, Bond R, Mulvenna M, et al. System Usability Scale Benchmarking for Digital Health Apps: Meta-analysis. JMIR Mhealth Uhealth 2022;10:e37290. [Crossref] [PubMed]
- Meneses-Gaya IC, Zuardi AW, Loureiro SR, et al. Psychometric properties of the Fagerström Test for Nicotine Dependence. J Bras Pneumol 2009;35:73-82. [Crossref] [PubMed]
- Budenz A, Coa K, Grenen E, et al. User Experiences With an SMS Text Messaging Program for Smoking Cessation: Qualitative Study. JMIR Form Res 2022;6:e32342. [Crossref] [PubMed]
- Douglas N, Free C. 'Someone batting in my corner': experiences of smoking-cessation support via text message. Br J Gen Pract 2013;63:e768-76. [Crossref] [PubMed]
- Naughton F, Jamison J, Sutton S. Attitudes towards SMS text message smoking cessation support: a qualitative study of pregnant smokers. Health Educ Res 2013;28:911-22. [Crossref] [PubMed]
- Coa KI, Wiseman KP, Higgins B, et al. Associations Between Engagement and Outcomes in the SmokefreeTXT Program: A Growth Mixture Modeling Analysis. Nicotine Tob Res 2019;21:663-9. [Crossref] [PubMed]
- Robinson CD, Wiseman KP, Webb Hooper M, et al. Engagement and Short-term Abstinence Outcomes Among Blacks and Whites in the National Cancer Institute's SmokefreeTXT Program. Nicotine Tob Res 2020;22:1622-6. [Crossref] [PubMed]
- Sloan M, Hopewell S, Coleman T, et al. Smoking Cessation Support by Text Message During Pregnancy: A Qualitative Study of Views and Experiences of the MiQuit Intervention. Nicotine Tob Res 2017;19:572-7. [Crossref] [PubMed]
- Cornelius ME, Wang TW, Jamal A, et al. Tobacco Product Use Among Adults - United States, 2019. MMWR Morb Mortal Wkly Rep 2020;69:1736-42. [Crossref] [PubMed]
- Naughton F, Jamison J, Boase S, et al. Randomized controlled trial to assess the short-term effectiveness of tailored web- and text-based facilitation of smoking cessation in primary care (iQuit in practice). Addiction 2014;109:1184-93. [Crossref] [PubMed]
- Texas Census QuickFacts - United States Census Bureau. [cited 2022 Oct 6]. Available online: https://www.census.gov/quickfacts/fact/table/TX/RHI725221#qf-headnote-b
Cite this article as: Klaff R, Tundealao S, Krenek B, Tamí-Maury I. Designing and pilot-testing SmokefreeSGM: a text-based smoking cessation intervention for sexual and gender minority groups. mHealth 2023;9:23.