A mobile diabetes management and educational system for type-2 diabetics in Saudi Arabia (SAED)
Introduction
Diabetes is one of the most common chronic diseases. As of 2013, approximately 382.8 million people between the ages of 20 and 79 are suffering with this condition worldwide. The worldwide costs for treatment of diabetes and its related complications in 2013 were estimated to be around $548 billion (1). The Kingdom of Saudi Arabia (KSA) has the seventh highest prevalence of diabetes in the world with over one-fifth of its population suffering with the disease (2).
Recently, there has been an increasing level in the use of mobile health technologies, especially for chronic disease management. A significant amount of research and pilot studies have evaluated the implementation of mobile health for chronic disease management particularly for diabetes management. The majority of these studies indicated that the use of mobile health technology in chronic disease management, in particular for diabetes management can significantly improve the diagnosis and management of the disease in both type-1 diabetic (T1D) and type-2 diabetic (T2D) patients which in turn improve their quality of life (3,4).
A typical smart mobile diabetes management system consists of several components of sensors, wireless technologies, GPS technologies and other functionalities designed to provide healthcare related services (5). These systems are cost effective solutions that can perform several functions such as monitoring the diabetes conditions, establishing a communication channel between the patient and the medical staff, enabling remote monitoring of the patient, record and maintain a database of patient’s medical history and enhancing the patient’s knowledge about the disease. In recent year, there have been numerous studies on the effectiveness of mobile diabetes management system (6-8). The clinical outcomes of these studies showed that an intervention using these mobile phone systems provided improved diabetes outcomes including reduced glycated hemoglobin (HbA1c) levels, with improved diabetes self-management, and enhanced knowledge about the disease.
In the KSA and the Gulf region in general there is an increasing prevalence of diabetes considered one of the highest globally, whilst at the same time, there has been an unprecedented increase in the use of smart mobile phones in the region.
To date, the benefits of mobile health technologies have not been fully realized in the Kingdom particularly for diabetes patients. Furthermore, to date there has been no reported clinical study to show the effectiveness of these systems for diabetes care in the Kingdom. In this paper, we present an intelligent mobile diabetes management system and tailored for T2D patients in Saudi Arabia designed with the aim of improving the self-management of the disease. The system is named SAED that means happiness in Arabic. We present the clinical results and outcomes of a pilot study of the SAED system conducted in the Tabuk region of the Kingdom. The paper is structured as follows: section ‘Mobile diabetes management system’ presents the architecture of the SAED system; section ‘Methods’ discusses the methodology adopted in our clinical trial followed by a discussion on the results in section ‘Results’; section ‘Discussion and conclusions’ concludes the paper.
Mobile diabetes management system
In this section, the SAED system architecture is presented followed by a discussion on the study design and methods in follow up section. A brief description of the SAED architecture is given here, a more detailed description of the architecture can be found in (9).
The SAED system architecture
Figure 1 illustrates the architecture of the SAED system. The system architecture was designed and developed by conducting a systematic requirement analysis and holding extensive discussions with the stakeholders in the United Kingdom and KSA. The SAED system compromises of three main components: (I) SAED mobile patient/healthcare provider component; (II) SAED intelligent diabetes management component; and (III) Diabetes educational module component.
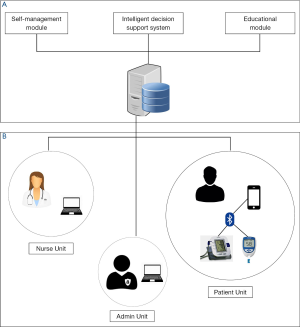
SAED mobile patient/healthcare provider component
This component is the mobile end of the system implemented using smart phone platform. This component covers most of the interaction units of the SAED system and further includes three sub-units. The diabetic patient unit consists of a smart mobile phone and a blood glucose measuring device enabled with Bluetooth. In this unit, the patient provides the measurements of his/her medical readings (e.g., by measuring the blood glucose level twice daily) as prescribed by the clinician. This data is transferred via a 3.5G/4G/LTE network to the SAED cloud server.
The Specialist diabetic nurse unit includes a friendly web interface and friendly mobile application version to the nurses or clinicians involved in the management of the patient. It presents a diabetes data management system which enables the clinician to monitor and communicate with diabetic patients remotely. It also provides the clinician certain controlled access features such as registering the patient, accessing the patient health records, providing reports, etc.
The admin unit is responsible for the systems repair and maintenance. It monitors the technical aspects of the system and is also responsible for maintaining the backup of the data collected via the system. All the 3 units feature a web interface which allows the users to interact with the system in an easy and efficient way.
SAED intelligent diabetes management component
This component represents the back end operations which mainly involve the process of collecting information or data and storing them in the database so that it can be used by various other modules such as the decision support systems, the educational module, the front end patient and clinician mobile unit, etc. This component includes Database module which stores information related to individual patients data and records related to the laboratory examination results (e.g., HbA1c test results) along with the specialist diabetic nurses comments on the diabetic readings standard. This unit also enables communication with the information system of the hospital where the SAED was deployed. The database was designed using the MySQL standard.
The Intelligent decision support is an important module of the SAED system designed to act as a clinical decision support system to the clinicians. The intelligent decision support system was designed to include the following features: a standardized format for presenting data and interventions, computerized alerts and reminders, a database of validated interventions for improving outcomes, patient data reports, work-flow tools, documentation templates, and an assessment tool to assess the patient‘s condition based on their readings.
Diabetes educational module
The diabetes educational module is aimed at empowering the diabetic patients with increased knowledge about diabetes which can further facilitate better self-management of the disease. Free SMS messages are sent to the patients from SAED web application to SAED mobile application. The educational program is structured based on a literature review of the earlier studies in neighboring countries of KSA particularly in Iraq (10) and Bahrain (8) and via extensive discussions and collaborations with medical staff from Saudi Arabia and based on guidelines for diabetes management provided by National Institute for Health & Care Excellence (NICE guidelines) in UK. The messages covered the following five diabetes education-related themes: (I) treatment; (II) blood glucose monitoring; (III) diabetes; (IV) complication awareness; (V) improvement of clinic attendance and diet. These themes were translated, programmed and embedded within the SAED system.
Methods
Study groups
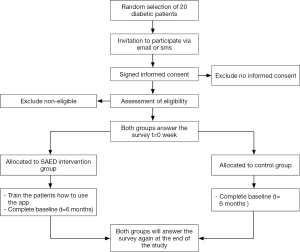
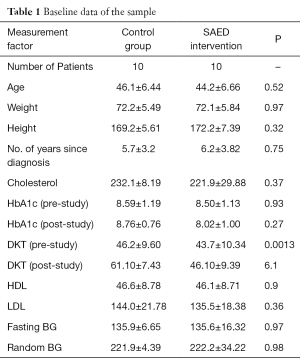
In order to test the SAED system, a small pilot study was conducted for 6 months period in the Tabuk region in KSA. The clinical trial was conducted with total of 20 participants. The patients were randomly segregated into two groups: Intervention Group and the Control Group with ten patients each. T2D patients of both genders with an age group between 20–65 years were selected. The baseline demographics of the patient sample are given in Table 1. A flowchart of the methodology followed during the clinical trial is illustrated in Figure 2.
Full table
SAED intervention group
In this group the patients were given the SAED system and were trained to operate and run the blood glucose device and to transmit the measurements using a smart phone downloaded with the SAED application. A general outline of the SAED intervention group’s clinical trial is given below:
- The schedule for blood glucose testing was set by the diabetic nurse for every patient. The typical setting used was: 2–3 times a day, usually before and after meals, on 2 or 3 different days in the week. The HbA1c testing was performed twice during the trial, at the beginning and at the end of the 6-month period;
- The patients used a device which measures the glucose levels and transmit the measured data via Bluetooth to the patient’s smart phone and then to the server located at the hospital in the Tabuk region;
- The patients accessed various services such as sending private messages or queries to the medical staff via a user friendly interface on their smart phone application;
- The medical staff monitored and reviewed the patient’s condition using the data collected and stored in the database to plan and advice on the treatment;
- Based on the blood glucose and HbA1c readings from the database the specialists made recommendations about the risk factors, lifestyle advice, and/or routine changes in pharmacological treatment in line with NICE and UK diabetes guidelines. This information was securely transmitted in real-time to the patients’ smart phone;
- SMS education program wherein the patients received weekly messages to keep them informed about diabetes and other related information.
Control group
The monitoring of the patients in the control group was done in the traditional way, i.e., the general practitioner and/or the practice nurse monitored the patients as done in normal circumstances. The patients received their advice on the management of diabetes according to the local guidelines as agreed between the primary and secondary care health careers.
Results
Data collection
Table 1 shows the baseline data of the patient sample of the clinical trial. From this, it can be seen that patients with diverse conditions were sampled. The characteristics of the patients considered in the tests are mentioned in the first column. The main features of patients considered are: age, height, weight, and period of diagnosis, cholesterol, HbA1c, knowledge about diabetes, HDL, LDL, and blood-glucose levels.
The data collection was carried out on two visits during the course of 6 months. The first visit was at the beginning of the 6-month period where the patient’s data was collected The standard procedures to collect the data were followed and the data collected included personal data like the patient’s personal information (i.e., name, birthday, and so on), medical history to record disease data such as smoking habits, presence of diabetes current pharmacological treatment and body mass index. The routine collection of the patient’s venous blood for the measurement of lipids, HbA1c, urine sample for albumin and retinal screening was also done. The baseline measures were reassessed at the end of the 6-month period.
Other medical and patients data types that were collected by the researcher and the medical staff included HbA1c, body weight, height, diabetes knowledge questionnaire and the system usability questionnaire. For the diabetes knowledge test the “Diabetes Knowledge Questionnaire” from (11) was adapted.
Results and analysis
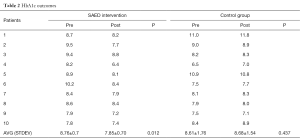
The two primary outcomes of the pilot study were considered are the HbA1c and the Diabetes knowledge test outcomes. At the beginning of the study, for the baseline information the demographic variables, glycosylated HbA1c, fasting plasma glucose (FPG), 2-hours post prandial test (2HPPT) measures were collected as the pre-test data. The diabetes knowledge was measured using the standard diabetes knowledge questionnaire as (pre and post)-test data for both groups. These factors were measured at the end of the study, i.e., after the 6-month period. To statistically analyse the results paired t-tests and correlation analysis were used. The analysis was carried out using the SPSS software for the data collected at the beginning and end of the trial study. Further, to analyse the effect of other factors including the educational level, linear regression methods were applied. For the collected data, the data was considered to be statistically significant when P<0.05. The outcomes of the HbA1c results of the two patients’ arms are summarized in Table 2.
Full table
From this table it can be seen that there is a significant reduction in the HbA1c outcomes at the end of the 6-month period for the SAED intervention group when compared with the control group. The mean baseline HbA1c for the intervention group was seen to be 8.76% (0.76% tolerance) and 8.61% for the control group. It is important to note that the mean baseline of the intervention group decreased to 7.85% from 8.76% at the end of the 6-month period. However, for the control group, the mean baseline slightly increased from 8.61% to 8.68%. Even at the individual level, most patients of the intervention group have shown considerable decrease in the HbA1c outcomes. Therefore, it is clear that the intervention group has shown better results than the control group. Similarly, it can be seen in the Table 3 below that there is a remarkable increase in the diabetes knowledge of the patients in the SAED intervention group over the control group who followed the traditional methodology of patient monitoring.

Full table
Furthermore, the mean baseline for the intervention group which was at 46.20% rise significantly to 61.10% in 6 months. The control group also showed slight increase, i.e., from 43.70% to 46.10%, however, when compared with the intervention group it is relatively much lower. It is also important to note that all patients in the intervention group have shown very good improvements in their diabetes knowledge, whereas in the control group there are high inconsistencies with each patient.
Discussion & conclusions
In this paper we presented a smart phone system designed and developed for self-management of T2D patients in the KSA. We also presented the details of a pilot study to test the effectiveness of this technology on improving the HbA1c levels and self-management of Saudi patients in the Tabuk region. The results indicated the clear improvement of HbA1c and the Diabetes knowledge outcomes of the intervention arm compared to the control group of the patents. The results are in agreement with other global studies that indicate the effectiveness of smart phone intervention of T2D patients. These results indicate that SAED system and approach can provide excellent assistance tool in improving the diabetic management of Saudi patient considering the high level of usage and penetration of smart phone technologies in the Kingdom. The SAED system can also provide the specialists with important medical information about each individual patient that are essential for the decision making process in their diabetes progress treatment plan. The integrated of educational tool can provide the diabetics patient with relevant information for improved diabetes management. This is especially important in remote region of KSA where healthcare facilities are still insufficient and lack specialist diabetic care that ensures effective medical intervention and care, where systems like SAED can provide such efficiency and effectiveness. Furthermore, the proper adaptation of system like SAED can provide an improved remote self-monitoring and management that can eventually reduce the burden of the relatively high levels of mortality rates due to this disease in this Kingdom and the region. Despite the successful pilot study, some challenges to SAED system exist. The pilot study was conducted on small patient population of 20 patients. In order to improve the robustness of the results, further large scale studies are needed to validate these results on larger patient population. Other obstacles that faced the team of the study included; he shortage of medical and engineering expertise in the region, poor infrastructure in terms of lack of specialist diabetic nurse and medical expertise and poor mobile technology infrastructure in the hospitals and training facilities for such new technologies. In addition to the existing social traditions that can resists in the adoption process new technologies such as mobile health solutions. These challenges can be alleviated by adopting some simple measures such as: (I) identify a clear m-health strategy for providing urgent solution to major medical challenges such as diabetes prevalence as part of the national e-healthcare plan in the Kingdom; (II) increase the awareness of using mobile health technologies amongst the medical community and their medical training; (III) improve the infrastructure for mobile technologies that can better facilitate the mobile health solutions in the healthcare sector; (IV) inclusion of m-health as part of the medical educational curriculum; (V) the need for the more public awareness plans on mobile health amongst diabetic patients in the Kingdom.
Acknowledgements
The first author would like to acknowledge the advice and assistance of Dr. Naif Almasloki, Consultant Dialectologist, King Fahd Hospital, Dammam, KSA and all clinical staff in the centre.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the institutional ethics board of Naval in Dammam and informed consent was obtained from all patients.
References
- International Diabetes Federation. IDF Diabetes Atlas. 6 edition. Brussels: International Diabetes Federation, 2014.
- Al-Elq AH. Current practice in the management of patients with type 2 diabetes mellitus in Saudi Arabia. Saudi Med J 2009;30:1551-6. [PubMed]
- Istepanian RS, Zitouni K, Harry D, et al. Evaluation of a mobile phone telemonitoring system for glycaemic control in patients with diabetes. J Telemed Telecare 2009;15:125-8. [Crossref] [PubMed]
- El Khaddar MA, Harroud H, Boulmalf M, et al. Emerging wireless technologies in e-health trends, challenges, and framework design issues. Tangier: Multimedia Computing and Systems (ICMCS), 2012 International Conference on, 2012:440-5.
- Istepanian RS, Laxminarayan S, Pattichis CS. editors. M-health: emerging mobile health systems. Berlin: Springer, 2006.
- Liang X, Wang Q, Yang X, et al. Effect of mobile phone intervention for diabetes on glycaemic control: a meta-analysis. Diabet Med 2011;28:455-63. [Crossref] [PubMed]
- Yoon KH, Kim HS. A short message service by cellular phone in type 2 diabetic patients for 12 months. Diabetes Res Clin Pract 2008;79:256-61. [Crossref] [PubMed]
- Hussein WI, Hasan K, Jaradat AA. Effectiveness of mobile phone short message service on diabetes mellitus management; the SMS-DM study. Diabetes Res Clin Pract 2011;94:e24-6. [Crossref] [PubMed]
- Alotaibi M, Istepanian RS, Sungoor A, et al. An intelligent mobile diabetes management and educational system for Saudi Arabia: System architecture. Valencia: IEEE-EMBS International Conference on Biomedical and Health Informatics (BHI), 2014:29-32.
- Haddad NS, Istepanian R, Philip N, et al. A feasibility study of mobile phone text messaging to support education and management of type 2 diabetes in Iraq. Diabetes Technol Ther 2014;16:454-9. [Crossref] [PubMed]
- Garcia AA, Villagomez ET, Brown SA, et al. The Starr County Diabetes Education Study: development of the Spanish-language diabetes knowledge questionnaire. Diabetes Care 2001;24:16-21. [Crossref] [PubMed]
Cite this article as: Alotaibi MM, Istepanian R, Philip N. A mobile diabetes management and educational system for type-2 diabetics in Saudi Arabia (SAED). mHealth 2016;2:33.