
Economic evaluation of a mobile phone text-message intervention for Australian adults with type 2 diabetes
Highlight box
Key findings
• The cost of delivering a text message intervention to people with type 2 diabetes in Australia was AU$36 per participant. The intervention had a 33% probability of being effective and cost-saving based on glycated hemoglobin outcomes. It had a 24% probability of being cost-effective based on quality adjusted life year outcomes.
What is known and what is new?
• Text message programs have been shown to improve the health outcomes for people with type 2 diabetes. Our study is the first to provide evidence on the cost-effectiveness of a text message intervention to improve the health of people with type 2 diabetes in Australia.
What is the implication, and what should change now?
• There is the need for new cost-effective treatment strategies due to the significant economic burden from type 2 diabetes. Research is needed to examine the costs and cost-effectiveness of the intervention with key populations.
Introduction
Diabetes prevalence in Australia is rising, having almost tripled between 2000 and 2020 with an estimated 1.3 million (1 in 20) people with the disease, which is reported to be an underestimation of true prevalence (1). Accounting for around 90% of all diabetes cases (2), type 2 diabetes attributed 2.3% of the total burden of disease in Australia in 2018 (3), being ranked the 12th leading contributor to disease burden (1) making it a public health concern that will worsen if rates continue to increase by a further 25% in 2030 and 51% by 2045 as predicted (2). The total burden of type 2 diabetes is largely attributable to modifiable risk factors such as overweight and obesity (36%), diet (19%), physical inactivity (13.8%) and tobacco use (2%) (1). Self-management education for adults with type 2 diabetes has been shown to improve health outcomes (4), however access and attendance in Australia is poor (5,6), and only half of those with the disease meet the recommendations for type 2 diabetes management (7).
The economic burden from type 2 diabetes on the healthcare system is significant (8) costing the Australian government $1.9bn in 2018–2019 (1), with the largest expenditure attributed to government subsidized medications (44%) and hospital services (40%) (9). Predictions show that for the hospital system in New South Wales (NSW) alone, Australia’s most populated state, costs are expected to become unsustainable totaling $21.7bn over the next decade if no changes to current practices occur (10). Reductions in glycated hemoglobin (HbA1c) have been shown to delay the onset and slow the progression of type 2 diabetes, and reduce diabetes-related complications and death (11). To better support people with type 2 diabetes manage their condition, and reduce the economic burden on the Australian health system, new cost-effective treatment strategies are required (12,13).
Text-message programs for people with type 2 diabetes have been shown to improve health outcomes and self-management behaviors, are relatively inexpensive and provide a highly accessible mode of communication with the potential to address health disparities in diabetes care (14). A meta-analysis of 1,701 participants with type 2 diabetes receiving unidirectional text-messages showed a significant reduction in HbA1c of 0.38% (15), and a recent review on text-message programs for people with type 2 diabetes demonstrated a consistent moderate effect size on improvements in HbA1c (14). Despite this, text-message programs are an underutilized adjunct to clinical care (14), and there is no evidence on their cost-effectiveness or costs of scale-up to a population level in the Australian context. Economic evidence is crucial to inform decision makers which interventions represent value for money to ensure limited health care budgets are well spent (16,17). In 2017–2018 we conducted a pragmatic 2-armed, parallel, non-blinded randomized controlled trial (RCT) to determine the effectiveness of a 6-month text-message intervention (DTEXT: ACTRN12617000416392) compared to usual care, for people with type 2 diabetes (18). The intervention was highly accepted and resulted in significant improvements in consumption of vegetables, fruit and sweet discretionary foods, and a non-significant trend for improved HbA1c (19). The present study aimed to determine the cost-effectiveness and cost-utility of DTEXT to improve HbA1c and self-management behaviors for Australian adults with type 2 diabetes. We present the following article in accordance with the Consolidated Health Economic Evaluation Reporting Standards (CHEERS) reporting checklist (20) (available at https://mhealth.amegroups.com/article/view/10.21037/mhealth-22-26/rc).
Methods
We conducted a within-trial economic evaluation on the RCT to determine the cost-effectiveness and cost-utility of DTEXT (intervention arm), compared to usual care only (control arm), on diabetes management for Australian adults with type 2 diabetes. A detailed study protocol for DTEXT has been published elsewhere (18). Whilst the observed difference in HbA1c between the intervention and control groups was non-significant, we proceeded with the planned economic evaluation, including a cost-utility analysis, to determine if the intervention could be cost saving, and to quantify the probability of it being cost-effective. A health funder plus patient perspective was used for the evaluation as costs are borne by both the Australian health system and the person living with type 2 diabetes in terms of out-of-pocket healthcare costs.
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the University of Wollongong & Illawarra Shoalhaven Local Health District Human Research Ethics Committee (Health and Medical) (No. 2016/343) and informed consent was taken from all individual participants.
Subjects
Participants were community dwelling residents of New South Wales (NSW), Australia, with type 2 diabetes and HbA1c ≥53 mmol/mol (7%), aged ≥18 years, who were able to read and speak English, could provide written informed consent, owned a mobile phone and were not pregnant. Medical clearance was obtained from participants’ doctors. Recruitment occurred primarily through Facebook and a mass mail-out, but also included referral from health professionals and advertisements in newspaper, radio and community noticeboards (21).
Intervention and control arms
DTEXT intervention arm received a mobile phone text-message program providing six months of self-management behavior change and diabetes care support. The text-messages were developed by an expert panel, with appropriate readability and using the behavior change theory and technique taxonomy (18). Text-messages were unidirectional and semi-personalized, with delivery occurring daily for months 1–3, and four times per week for months 4–6. The message content included nutrition, physical activity, diabetes care, weight management, medication adherence and smoking cessation. The control arm received usual care from their treating doctor and associated health professionals. HbA1c and quality of life measures for both arms were taken at baseline, 3 and 6 months. All participants could withdraw from the study at any time by texting STOP.
Health outcome measures
HbA1c
The primary outcome of the DTEXT study was the difference in HbA1c between intervention and control at 6 months, determined by non-fasting blood test taken at the participant’s local pathology collection center.
Quality-adjusted life years (QALYs)
Quality of life was determined by the Short Form 12-item Health Survey (SF12v2), a multi-purpose survey measuring quality of life by functional health and wellbeing (22). To calculate QALYs, health utilities were converted to a preference-based measure of quality of life, the Short Form Six Dimension (SF-6D) health index (23), using the UK valuation algorithm (24). QALYs over the 6-month period of the trial were determined for intervention and control arms using utilities at baseline, 3 and 6 months. QALYs were summed over each 3-month period from duration of time multiplied by mean utility. For the 6% of participants with a missing utility measurement at 3 months, QALYs were calculated from the baseline and 6-month measurements. If participants were missing a baseline or 6-month measure they were excluded from the analysis.
Measurement and valuation of resource use
The cost of delivering the intervention and the healthcare costs of participants were collected and valued in 2018 Australian dollars (AUD). Conversions to an international dollar value (INT$) were calculated using the World Bank PPP conversion factor (25). As the intervention ran for less than one year, discounting was not applied. The cost of delivering the intervention included text-message costs, staffing costs and consumables which were calculated to determine total intervention costs for the trial, and a cost per participant. Out-of-hospital costs of health services and medications were determined by individual patient data linkage to Medicare Benefits Schedule (MBS) and Pharmaceutical Benefits Scheme (PBS) data. Medicare is a universal healthcare system that provides Government subsidies for out-of-hospital health care services, tests and interventions. The MBS is a listing of the Medicare health care services subsidized by the Australian federal government. The PBS claims data lists all the medicines dispensed to patients subsidized by the Australian government. All Australian residents who hold a Medicare card are eligible for the PBS. All MBS items, and PBS items relevant to type 2 diabetes management (26) were included in the analysis. Items were checked by experts in the field for relevancy in the analysis. Health care costs relating to hospital admissions were not captured as the short 6-month duration of DTEXT was not anticipated to impact on these.
Economic evaluation
The economic evaluation included a cost-effectiveness analysis and cost-utility analysis. The cost-effectiveness analysis utilized the intervention mean costs and health outcomes of each trial arm to determine the incremental costs per 11 mmol/mol (1%) reduced HbA1c, compared to usual care. The cost-utility analysis estimated the incremental cost per QALY gained.
Incremental cost-effectiveness ratios (ICERs) were calculated for HbA1c and QALY outcomes. Each ICER was calculated by dividing the difference in per participant mean cost (intervention minus control) by the difference in outcome measures.
Bootstrapping techniques used 1,000 replications with replacement to estimate the joint uncertainty of costs and health outcomes, which were plotted on an incremental cost-effectiveness plane. Cost-effectiveness acceptability curves (CEAC) determined the probability that the DTEXT intervention was cost-effective over a range of willingness to pay thresholds.
A scenario analysis was conducted to determine if the ICERs and probability of cost-effectiveness changed markedly when the cost of text-messages was adjusted to reflect the current market costs of text-message (7 cents rather than 12 cents at the time of the trial) and consumables specific to the study were removed (a NSW Health database storage fee specific to this study that would not be charged if the intervention was translated into practice).
Results
Three hundred and ninety-five participants (mean age 62 years, 50% male) were randomized to either the intervention (n=197) or control (n=198) arm of the DTEXT intervention. The majority (88%, n=348) of participant data was included in the economic evaluation [intervention (n=176), control (n=172)], with exclusions due to consent not provided for MBS/PBS data linkage (2%); and missing data for the 6-month HbA1c measures (6%) and the SF12v2 questionnaire (4%). Baseline demographics, clinical measures, and health utility scores for participants of the economic evaluation were similar between the intervention and control arms (Table 1).
Table 1
| Outcome | Control (n=172) | Intervention (n=176) |
|---|---|---|
| Demographic | ||
| Males | 91 (52.9) | 84 (47.7) |
| Age (years) | 62.83±10.29 | 62.10±9.59 |
| Born in Australia | 129 (75.0) | 129 (73.3) |
| Aboriginal or Torres Strait Islander origin† | 9 (5.2) | 9 (5.1) |
| Education level of year 12 or less | 50 (29.1) | 58 (33.0) |
| Paid employment | 67 (39.0) | 61 (34.7) |
| Smoker | 12 (7.0) | 11 (6.3) |
| Taking medication for diabetes† | 169 (98.3) | 167 (94.9) |
| Self-reported health fair/poor | 67 (39.0) | 61 (34.7) |
| Objective clinical measures | ||
| HbA1c [mmol/mol (%)] | 66 (8.19)±1.15 | 66 (8.16)±1.16 |
| Total cholesterol (mmol/L)† | 4.10±1.11 | 4.16±1.13 |
| Body mass index (kg/m2)† | 33.88±7.46 | 33.42±6.41 |
| Health Utility Score—SF12v2 | ||
| Quality of life (mental)† | 50.28±11.27 | 50.42±10.51 |
| Quality of life (physical)† | 41.84±11.11 | 42.06±10.89 |
| Excluded from analysis | ||
| Participants with missing data | 26 (15.1) | 21 (11.9) |
Data are expressed as n (%) or mean ± SD. †, n<348. HbA1c, glycated hemoglobin; SD, standard deviation.
Costs and outcomes
The total cost of delivering the 6-month text-message intervention (DTEXT) to 197 participants was AU$7,106 (INT$4,827), which equates to AU$36 (INT$24) per participant. The delivery of text-messages contributed to almost half of the costs. There were no intervention costs associated with the control arm as this arm received usual care only (Table 2).
Table 2
| Resource item | Resource use | Units | Unit cost (AUD) | Cost (AUD) | Source of unit costing |
|---|---|---|---|---|---|
| Equipment and overhead | |||||
| Delivery of text messages | 135 messages per participant (n=197) | 26,595 | 0.12 | 3,191 | Message media |
| Account activation and licence fee | One-off payment | 1 | 49 | 49 | Message media |
| Access fee | Monthly basis | 6 | 50 | 300 | Message media |
| Dedicated mobile number activation | One-off payment | 1 | 150 | 150 | Message media |
| Dedicated mobile number/alpha tag licence fee | Monthly basis | 6 | 25 | 150 | Message media |
| Maintenance of text message database and delivery system | Monthly fee for IT server | 6 | 197 | 1,182 | NSW Health internal cost |
| Database storage fee | One-off payment | 1 | 1,989 | 1,989 | NSW Health internal cost |
| Staff time | |||||
| Data entry | Administrative task, 1 min per participant | 197 | 0.48 | 95 | NSW Health award for Administration Officer (level 3) at hourly rate of $29.02 |
| Total intervention cost | – | – | – | 7,106 | – |
| Total cost per participant | – | – | – | 36 | – |
DTEXT, text message intervention; AUD, Costs in Australian dollars for the year 2018; IT, information technology; Min, minutes.
The mean healthcare costs over 6 months (health funder plus patient out-of-pocket expenses) for the intervention and control arms were similar, costing AU$1,982 (INT$1,346) and AU$1,974 (INT$1,341) per participant respectively. The mean difference in HbA1c at 6 months between the intervention and control arms was 1.1 mmol/mol (95% CI: −1.4 to 4.5), 0.1% (95% CI: −0.13 to 0.41), favoring the intervention group. The corresponding incremental QALY was −0.004 (95% CI: −0.02 to 0.01), favoring the control arm (Table 3).
Table 3
| Costs and health outcomes | Mean (bootstrapped 95% CI) | AUD per health gain (bootstrapped 95% CI) | |||
|---|---|---|---|---|---|
| Control (n=172) | Intervention (n=176) | Incremental† | ICER | ||
| Costs | |||||
| Intervention costs (AUD) | 0 | 36 | |||
| Patient costs (AUD) | 303 | 273 | |||
| Health funder cost (AUD) | 1,671 (1,512, 1,884) | 1,709 (1,530, 1,903) | 38 (−207, 302) | ||
| Health funder plus patient cost (AUD) | 1,974 (1,808, 2,145) | 1,982 (1,789, 2,187) | 8 (−250, 289) | ||
| Total costs (AUD) | 1,974 (1,808, 2,145) | 2,018 (1,825, 2,223) | 44 (−214, 325) | ||
| Health outcomes | |||||
| HbA1c (mmol/mol) at 6 months | 65 (63, 68) | 64 (62, 66) | 1.1 (−1.4, 4.5) | 311 (−10,800, 5,559) per 11 mmol/mol HbA1c reduction | |
| HbA1c (%) at 6 months | 8.1 (7.94, 8.33) | 8.0 (7.81, 8.17) | 0.1 (−0.13, 0.41) | 311 (−10,800, 5,559) per 1% HbA1c reduction | |
| QALYs over 6 months | 0.367 (0.36, 0.38) | 0.363 (0.35, 0.37) | −0.004 (−0.02, 0.01) | Not calculated* | |
†, intervention minus control, except for HbA1c which is control minus intervention to allow for calculation of HbA1c percentage points saved; *, an ICER could not be calculated as mean QALYs were higher in the control group than in the intervention group. DTEXT, text-message intervention; ICER, incremental cost effectiveness ratio; AUD, Australian Dollars for the year 2018; CI, confidence interval; HbA1c, glycated hemoglobin; QALY, quality adjusted life year.
Economic evaluation
Cost-effectiveness
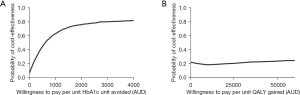
The ICER at 6 months was AU$311 (INT$211) per 11 mmol/mol (1%) reduced HbA1c (95% CI: −10,800 to 5,559). Most bootstrapped replicates fell in the northeast quadrant, indicating that the intervention was more effective and more costly (Figure 1). The probability of DTEXT being more effective and cost-saving than usual care for improvements in HbA1c was 33% (Figure 1A). The CEAC (Figure 2A) showed a 75% probability of the intervention being cost effective at a nominal willingness to pay threshold of AU$1,800 (INT$1,223) per 11 mmol/mol (1%) reduced HbA1c, compared to usual care.

Cost-utility
A mean ICER for QALYs was not calculated as the control arm dominated the intervention for this measure. The probability of DTEXT being more effective and cost-saving than usual care for QALYs was 15% (Figure 1B), with mean QALYs slightly higher in the control arm than the intervention. The CEAC for QALYs (Figure 2B) showed a 24% probability that the intervention was cost-effective compared with usual care at the commonly used willingness to pay threshold in Australia of AU$50,000 (27) per QALY gained.
Scenario analysis
Based on more realistic costs of text-messaging reflecting the current market cost per text-message and no study database storage fees, scenario analysis indicated the cost per participant reduced from AU$36 (INT$24) to AU$13 (INT$9). The corresponding ICER reduced from the base case value of AU$311 (INT$211) per 11 mmol/mol (1%) reduced HbA1c over the 6-month period to AU$151 (INT$103). The probability of the intervention being effective and cost-saving increased from 33% to 38%. The probability that the intervention was cost-effective at a willingness to pay threshold of AU$50,000 per QALY increased slightly to 25%. A mean ICER for QALYs was not calculated as the control arm dominated the intervention.
Discussion
This health economic evaluation provides the first evidence on the costs and cost-effectiveness of a text-message intervention for people with type 2 diabetes in Australia. The cost of delivering DTEXT intervention was low at AU$36 (INT$24) per participant in study conditions, and AU$13 (INT$9) per participant based on scenario analysis. The highly accepted DTEXT text-message intervention providing self-management support and diabetes care for Australian adults with type 2 diabetes, showed a non-significant trend to reduce HbA1c compared to usual care, and statistically significant improvements in the type 2 diabetes nutritional risk factors of vegetables, fruit and sweet discretionary food consumption (19). The cost-effectiveness analysis showed that DTEXT had a 33–38% probability of being effective and cost saving over 6 months, with an ICER of AU$311 (INT$211)–AU$151 (INT$103) per 11 mmol/mol (1%) HbA1c reduction. DTEXT did not result in higher QALYs than the control, and had only a 24–25% probability of being cost-effective based on usual cost/QALY thresholds.
The evidence for economic evaluations of type 2 diabetes text-message interventions is limited (28), making comparisons with DTEXT outcomes challenging. To the best of our knowledge, no similar studies exist, except for one from Bangladesh that reported a text-message program delivery cost of INT$24 per patient, an incremental cost of INT$38 per 11 mmol/mol (1%) reduction in HbA1c and an ICER of INT$2,406 per QALY gained (29). This represents a similar cost of intervention delivery as DTEXT and a similar incremental cost per HbA1c reduction as our scenario modelling outcome. The ICER for QALYs for the Bangladesh study was reported to be cost-effective, however in our study the intervention had only a 24% probability of being cost-effective for the QALY outcome. This may be explained by our study being powered to detect HbA1c outcomes and not costing or quality of life measures (4), or that 6 months duration is not long enough to show significant improvements in diabetes complications (30) and complex constructs (29), which affect quality of life. More economic evaluations of text-message programs for people with type 2 diabetes are required to provide an adequate comparison of DTEXT outcomes.
As the cost-effectiveness comparison data for DTEXT is currently limited, considering the costs required for scale-up by the Australian Government may be useful. Our scenario modelling suggests this would cost the health services AU$13/person to provide 6 months of supportive text-messages, which is considerably cheaper than the government cost to cover one single standard general practitioner (AU$38.75) or allied health (AU$54.60) consultation (31). Interventions similar to DTEXT have been deemed scalable and most cost-effective when implemented at a large or national scale (32), which may further reduce DTEXT costs and support scale-up potential. It has been shown that programs that reduce HbA1c over the short term can lead to substantial savings, and that reductions in HbA1c of less than 11 mmol/mol (1%) still lead to costs-savings in health care (33). Single technology interventions such as DTEXT have reported acceptability due to being user friendly, with lower costs, and less complexity (16).
Our study had several strengths including its robustness using costs and outcomes from the largest randomized controlled trial (19) to date of participants for a type 2 diabetes text-message intervention on self-management strategies and diabetes care. The use of data linkage from the MBS/PBS provided accurate and objective measures of out-of-hospital health care utilization and costs which is currently lacking from the existing evidence (29). The pragmatic study design enabled an economic evaluation to be conducted in a real-world setting allowing for realistic assessment of population scale-up suitability (34). The text-message program was highly accepted by people with type 2 diabetes, an important consideration for implementation (4). The study limitations include the short 6-month follow-up period, however, HbA1c improvement (32) and long-term health outcomes and cost savings may be observed at longer follow-up (29). Modelling was not undertaken which may have provided an indication of long term effects, and validated disease progression models offer the best prospective for evaluating the cost-effectiveness of self-management support interventions (4). Furthermore, our intervention costing did not include costs beyond the timeframe of the trial, such as IT system upgrades and text message adaptations. While it is not appropriate for an economic evaluation to include costs incurred beyond the timeframe that the outcomes are measured, it may be important to consider these costs when integrating such a program in the health system.
Our findings have implications for future research and the Australian health system. Important future research might investigate if the cost-effectiveness of DTEXT differed among different aged participants, culturally and linguistically diverse populations, those with differently controlled HbA1c or those with newly diagnosed type 2 diabetes as it is plausible that its effectiveness and cost-effectiveness is heterogenous. For example, it is suggested that the early years of living with the disease is the optimal time to support behavior change and educational reinforcement (14), can provide greater treatment effects (14) and benefits to the individual and health system (35). Depending on its effectiveness and cost-effectiveness in key population groups, implementation of DTEXT may be worth consideration in targeted populations as the simple and low cost delivery of automated text-messages can deliver ongoing support and provide an equitable adjunct to usual care. From a health system perspective, DTEXT is an affordable intervention that is feasible to deliver, with a 33–38% probability of being cost-saving and effective. Moreover, the intervention was highly accepted by community dwelling adults with type 2 diabetes (19) can reach people anywhere in their everyday lives and benefit care (32). Future research prior to implementation would also have to consider cultural and linguistically diverse populations and the need to adapt the content for people of different health literacy.
Conclusions
This study provides high quality evidence to the limited pool of health economics research on text-message programs for people with type 2 diabetes. The intervention was low cost but had low to moderate probability of being cost-saving and effective. In light of its affordability and acceptability, there is potential for a targeted approach to its implementation as an adjunct to usual care but further research is needed to examine its effectiveness and cost-effectiveness in key populations.
Acknowledgments
We would like to thank the DTEXT Advisory Committee’s for their guidance in the study design; and Vanessa Jackson, Dr. Jenny Norman and Tania Starr from the Illawarra Shoalhaven Local Health District Health Promotion Service for their administrative support and advice.
Funding: This work was supported by a NSW Ministry of Health Translational Research Grants Scheme (Round 1: 2016, Project ID: 23). The funding source had no involvement in the study design; collection, analysis or interpretation of data; writing of the report; or decision to submit the article for publication.
Footnote
Reporting Checklist: The authors have completed the Consolidated Health Economic Evaluation Reporting Standards (CHEERS) reporting checklist. Available at https://mhealth.amegroups.com/article/view/10.21037/mhealth-22-26/rc
Data Sharing Statement: Available at https://mhealth.amegroups.com/article/view/10.21037/mhealth-22-26/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://mhealth.amegroups.com/article/view/10.21037/mhealth-22-26/coif). AAG received salary support through an Emerging Leader 1 Investigator Grant (No. AAP1173784) from the Australian National Health and Medical Research Council and chose to spend her work time on this study. All other authors report that this work was supported by a NSW Ministry of Health Translational Research Grants Scheme (Round 1: 2016, Project ID: 23). AAK is supported by an Australian National Health and Medical Research Council Postgraduate research stipend (No. APP1169039) and salary funding (No. APP1101675) for research projects unrelated to this project. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the University of Wollongong & Illawarra Shoalhaven Local Health District Human Research Ethics Committee (Health and Medical) (No. 2016/343) and informed consent was taken from all individual participants.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Australian Institute of Health and Welfare. Diabetes: Australian Facts. 2022.
- Saeedi P, Petersohn I, Salpea P, et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res Clin Pract 2019;157:107843.
- Australian Institute of Health and Welfare. Australian Burden of Disease Study: impact and causes of illness and death in Australia 2018. Australian Burden of Disease Study series n0.23. Cat. no. BOD 29. Canberra: AIHW, 2021.
- Teljeur C, Moran PS, Walshe S, et al. Economic evaluation of chronic disease self-management for people with diabetes: a systematic review. Diabet Med 2017;34:1040-9. [Crossref] [PubMed]
- Economics DA. Benefits of Credentialled Diabetes Educators to people with diabetes and Australia. Canberra, ACT: Australian Diabetes Educators Association Limited, 2014.
- Australian Institute of Health and Welfare. Diabetes indicators for the Australian National Diabetes Strategy 2016–2020. 2018.
- Sainsbury E, Shi Y, Flack J, et al. Burden of Diabetes in Australia: It's time for more action. Preliminary Report. 2018 July.
- Friel KM, Gillespie P, Coates V, et al. Estimating and examining the costs of inpatient diabetes care in an Irish Public Hospital. Diabet Med 2022;39:e14753. [Crossref] [PubMed]
- Australian Institute of Health and Welfare. Disease expenditure in Australia 2018-19. Cat. no: HWE81. 2021.
- HealthStats NSW. Diabetes prevalence in adults 2020 [HealthStats NSW]. Available online: http://www.healthstats.nsw.gov.au/Indicator/dia_prev_age/dia_prev_age?&topic=Diabetes%20&topic1=topic_dia&code=dia[_]
- Stratton IM, Adler AI, Neil HA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ 2000;321:405-12. [Crossref] [PubMed]
- Varney JE, Liew D, Weiland TJ, et al. The cost-effectiveness of hospital-based telephone coaching for people with type 2 diabetes: a 10 year modelling analysis. BMC Health Serv Res 2016;16:521. [Crossref] [PubMed]
- McKinsey and Company. Evaluation Report of the Diabetes Care Project. 2015.
- Whittemore R, Siverly L, Wischik DL, et al. An Umbrella Review of Text Message Programs for Adults With Type 2 Diabetes. Diabetes Educ 2020;46:514-26. [Crossref] [PubMed]
- Haider R, Sudini L, Chow CK, et al. Mobile phone text messaging in improving glycaemic control for patients with type 2 diabetes mellitus: A systematic review and meta-analysis. Diabetes Res Clin Pract 2019;150:27-37. [Crossref] [PubMed]
- Rinaldi G, Hijazi A, Haghparast-Bidgoli H. Cost and cost-effectiveness of mHealth interventions for the prevention and control of type 2 diabetes mellitus: A systematic review. Diabetes Res Clin Pract 2020;162:108084. [Crossref] [PubMed]
- Palmer AJ, Holt RIG. Using health economics to count the cost of diabetes. Diabet Med 2019;36:925-6. [Crossref] [PubMed]
- Waller K, Furber S, Bauman A, et al. DTEXT - text messaging intervention to improve outcomes of people with type 2 diabetes: protocol for randomised controlled trial and cost-effectiveness analysis. BMC Public Health 2019;19:262. [Crossref] [PubMed]
- Waller K, Furber S, Bauman A, et al. Effectiveness and acceptability of a text message intervention (DTEXT) on HbA1c and self-management for people with type 2 diabetes. A randomized controlled trial. Patient Educ Couns 2021;104:1736-44. [Crossref] [PubMed]
- Husereau D, Drummond M, Augustovski F, et al. Consolidated Health Economic Evaluation Reporting Standards 2022 (CHEERS 2022) Statement: Updated Reporting Guidance for Health Economic Evaluations. Value Health 2022;25:3-9. [Crossref] [PubMed]
- Waller K, Furber S, Cook R, et al. Effectiveness and costs of strategies to recruit Australian adults with type 2 diabetes into a text message intervention (DTEXT) study. Public Health Res Pract 2022;32:31232110. [Crossref] [PubMed]
- Maruish ME, editor. User’s manual for the SF-12v2 Health Survey. 3rd ed. Lincoln, RI: QualityMetric Incorporated, 2012.
- Brazier J, Roberts J, Deverill M. The estimation of a preference-based measure of health from the SF-36. J Health Econ 2002;21:271-92. [Crossref] [PubMed]
- Brazier JE, Roberts J. The estimation of a preference-based measure of health from the SF-12. Med Care 2004;42:851-9. [Crossref] [PubMed]
- The World Bank. PPP Conversion Factor, GDP (LCU per international $) - Australia, United States 2021. Available online: https://data.worldbank.org/indicator/PA.NUS.PPP?end=2020&locations=AU-US&start=1990&view=chart
- The Royal Australian College of General Practitioners. General practice management of type 2 diabetes: 2016–18. East Melbourne, Vic: RACGP; 2016.
- Grosse SD. Assessing cost-effectiveness in healthcare: history of the $50,000 per QALY threshold. Expert Rev Pharmacoecon Outcomes Res 2008;8:165-78. [Crossref] [PubMed]
- de la Torre-Díez I, López-Coronado M, Vaca C, et al. Cost-utility and cost-effectiveness studies of telemedicine, electronic, and mobile health systems in the literature: a systematic review. Telemed J E Health 2015;21:81-5. [Crossref] [PubMed]
- Islam SMS, Peiffer R, Chow CK, et al. Cost-effectiveness of a mobile-phone text messaging intervention on type 2 diabetes—A randomized-controlled trial. Health Policy Technol 2020;9:79-85. [Crossref]
- Beaudet A, Clegg J, Thuresson PO, et al. Review of utility values for economic modeling in type 2 diabetes. Value Health 2014;17:462-70. [Crossref] [PubMed]
- Health AGDo. Medicare Benefits Schedule Book. 2021.
- Dobson R, Whittaker R, Jiang Y, et al. Long-term follow-up of a randomized controlled trial of a text-message diabetes self-management support programme, SMS4BG. Diabet Med 2020;37:311-8. [Crossref] [PubMed]
- Bansal M, Shah M, Reilly B, et al. Impact of Reducing Glycated Hemoglobin on Healthcare Costs Among a Population with Uncontrolled Diabetes. Appl Health Econ Health Policy 2018;16:675-84. [Crossref] [PubMed]
- McCrabb S, Lane C, Hall A, et al. Scaling-up evidence-based obesity interventions: A systematic review assessing intervention adaptations and effectiveness and quantifying the scale-up penalty. Obes Rev 2019;20:964-82. [Crossref] [PubMed]
- Meyerowitz-Katz G, Seelan S, Gaur P, et al. Detecting the hidden burden of pre-diabetes and diabetes in Western Sydney. Diabetes Res Clin Pract 2019;151:247-51. [Crossref] [PubMed]
Cite this article as: Waller KA, Killedar AA, Furber SE, Tan EJ, Gibson AA, Bauman AE, Hayes AJ. Economic evaluation of a mobile phone text-message intervention for Australian adults with type 2 diabetes. mHealth 2023;9:12.


